Abstract
Background:
Resting oxygen consumption (VO2) is often estimated and frequently used to guide therapeutic decisions in symptomatic heart failure (HF) patients. The relationship between resting VO2 and symptomatic HF and the accuracy of estimations of VO2 in this population are unknown.
Methods and Results:
We performed a cross-sectional study of HF patients (n=691) and healthy controls (n=77). VO2 was measured using a metabolic cart and estimated VO2 was calculated using the Dehmer, LaFarge, and Bergstra formulas and the thermodilution method. The measured and estimated VO2 were compared, and the potential impact of estimations was determined. In the multivariable model, resting VO2 decreased with increasing NYHA class in a stepwise fashion (β NYHA class IV vs controls=−36 ml O2/min, P<0.001). Estimations of VO2 using derived equations diverged from measured values, particularly for patients with NYHA class IV limitations. The percent difference of measured VO2 versus estimated VO2 was greater than 25% in 39% (n=271), 25% (n=170), 82% (n=566), and 39% (n=271) of HF patients when using the Dehmer, LaFarge, Bergstra, and thermodilution-derived VO2 respectively.
Conclusions:
Resting VO2 decreases with increasing NYHA class and is lower than controls. Using estimations of VO2 to calculate CO may introduce clinically important error.
Keywords: heart failure, hemodynamics, diagnostic testing
Introduction
Compromise in the delivery of oxygenated blood, one of the central purposes of the cardiovascular system, is a defining limitation of heart failure (HF). Measurement of cardiac output (CO) is necessary to evaluate cardiac function and estimate clinical prognosis. As a result, CO is frequently used to guide therapeutic decisions centered on maximizing blood flow to vital organs, limiting systemic and pulmonary vascular resistance, and optimizing right and left ventricular stroke work. The Fick principle1 and thermodilution (Td) are the most commonly used methods to calculate CO. Td has been shown to be inaccurate in patients with tricuspid regurgitation (TR) and low CO, both of which are common in advanced heart failure patients.2–5 The Fick equation has been well validated and is calculated using measurements of oxygen consumption (VO2) and both arterial and venous oxygen contents.6 Measurement of VO2 requires either mass spectrometry of exhaled air using a Douglas bag or breath-by-breath analysis with a metabolic cart. An estimated VO2 is frequently substituted for the directly measured value due to lack of available equipment and time required for measurement by using one of several previously published formulas (Table 1).7–9
Table 1:
Formulas used to calculate estimated oxygen consumption
BSA, body surface area; yrs, years; SD, standard deviation; VO2, oxygen consumption.
| Authors, Year | Name | Equation | Developmental Cohort Summary |
|---|---|---|---|
| Dehmer, Firth, and Hills, 19708 | Dehmer | VO2 = 125 × BSA* | n = 108, mean age = 49 yrs (SD not provided), % male = 64, % cardiomyopathy = 9.0 |
| LaFarge and Miettinen, 19707 | LaFarge | VO2 = 138.1 − (11.49 × log age) + (0.378 × HR) × BSA* for men; VC2 = 138.1 − (17.04 × log age) + (0.378 × HR) × BSA for women | n = 879, mean age not provided, between 3–40 yrs, % male = 59, % cardiomyopathy not provided |
| Bergstra, van Dijk, Hillege, Lie and Mook, 19959 | Bergstra | VO2 = 157.3 × BSA* + 10 − (10.5 × log age) + 4.8 for men; VO2 = 157.3 × BSA* − (10.5 × log age) + 4.8 for women | n = 250, mean age 34.6 +/− 22.7 yrs, % male = 57, % cardiomyopathy not provided |
BSA calculated using the formula of Dubois16 with BSA (m2) = 0.007184 x weight (kg)0.425 x Height (cm)0.725
While it is more convenient to use assumed values for VO2, there is evidence that VO2 estimations may not be accurate.9–13 The most commonly used equations to estimate CO (Table 1) were created with patient populations that are different from the advanced HF populations for which estimations are frequently applied. For example, the study by Dehmer, Firth, and Hillis included <10% of patients with a cardiomyopathy.8 Several studies have documented the inaccuracies of using estimations of VO2 in a variety of patient populations including a group of heart failure patients with reduced ejection fraction.10–14 More importantly, the use of an inaccurate VO2 to calculate CO with the Fick equation could impact clinical decision making including organ allocation, as the cardiac index is incorporated into the new UNOS guidelines for transplant listing.15 Given the potential ramifications of inaccurately estimating VO2, it is critical to better understand the relationship between VO2 and HF and to determine if it is acceptable to use estimations in HF patients with varying symptomatology as defined by New York Heart Association (NYHA) functional class.
In this study, we sought to do the following: 1. evaluate resting VO2 in control patients and HF patients grouped by NYHA class, 2. compare the results of measured VO2 to estimated VO2 using commonly used formulas in HF patients, and 3. analyze the potential impact of errors due to estimation on clinical decisions.
Methods
Study Design and Patient Selection
We performed a cross-sectional study examining VO2 in HF patients and healthy controls. All HF patients who underwent resting VO2 assessment (ROCA) at the time of right heart catheterization at the University of Michigan from March 2011 to May 2015 were included. If a patient underwent ROCA more than once, only the initial assessment was included in the study. HF patients were excluded if they had incomplete data, prior transplant, or mechanical assist device. Healthy volunteers were recruited from the University of Michigan portal for clinical research to serve as controls prospectively.
Patient charts were reviewed for demographic and clinical characteristics including NYHA classification. All patients hospitalized for heart failure at the time of measurement were considered to be NYHA class IV.
VO2 Measurement and Calculation
Patients and controls underwent direct measurement of VO2 in a fasting, supine, and non-sedated state after a 10-minute resting period using a canopy hood and a breathing valve apparatus and the Vmax TM Encore® Metabolic Cart. Among those undergoing right heart catheterization, the assessment was completed immediately prior to the patient’s scheduled catheterization in the pre-procedure holding area. Estimated VO2 was calculated using the Dehmer, LaFarge, and Bergstra Formulas as shown in table 1.7–9 Additionally, VO2 determined for the HF patients who underwent catheterization using the Td method and the arteriovenous oxygen content difference. Body surface area (BSA) calculated using the formula previously published by Du Bois.16 PVR was calculated using the equation: (mean pulmonary artery pressure – pulmonary capillary wedge pressure)/cardiac output.
Statistical Analysis
The association of worsening HF functional status and VO2 was assessed with univariable and multivariable linear regression. In the analysis, measured VO2 was the dependent variable and NYHA functional class was the primary predictor. Control patients served as the reference for the NYHA functional class variable. We adjusted for confounders known to impact VO2 including age, sex, and BSA in the primary multivariable analysis.7–9, 13 Additional analysis was performed including using body mass index in place of BSA and inclusion of left ventricular ejection fraction (grouped by ejection fraction ≤40%, 40–50%, or ≥50%) and resting hemodynamic variables (right atrial pressure and pulmonary capillary wedge pressure) among those who underwent a cardiac catheterization. In the model including resting hemodynamic variables, the NYHA class IV patients were the reference group. The assumptions for multivariable linear regression were assessed including multicollinearity (correlation of the independent variables) and homoscedasticity (similar variation of residuals) by variance inflation factor analysis and residual plots respectively. Using the final multivariable regression model, posterior estimations of the marginal mean effect of NYHA class on measured VO2 was estimated using the Stata (v15) margins command. This estimation determines the predicted VO2 for a given NYHA class while additional variables in the model are held constant.
Measured VO2 was compared to estimated VO2 grouped by NYHA class and sex using the Dehmer, LaFarge, and Bergstra formulas as well as the Td VO2 for HF patients using paired Student t tests. Patients were again grouped by sex as gender has previously been shown to effect VO2.13
Bland-Altman plots were created to assess agreement and bias between measured VO2 versus Dehmer estimations for controls and NYHA class IV patients.17 We selected the Dehmer equation for the comparison because it is the equation used in our catheterization laboratory.
Percent differences of measured versus estimated VO2 in HF patients were calculated by the absolute difference of measured VO2 - estimated VO2 / measured VO2 × 100 using the Dehmer, LaFarge, Bergstra, and Td derived estimated VO2. The potential clinical effect of errors in estimation of VO2 was simulated by comparing the pulmonary vascular resistance (PVR) calculated by using the measured VO2 versus the estimated VO2 calculated by the Dehmer, Bergstra, and LaFarge formulas in HF patients and identifying patients who had a permissible PVR using estimated VO2 but impermissible using measured VO2. A PVR of 3.0 Woods units was selected as the cutoff in line with current guideline recommendations to determine which candidates for heart transplantation require a vasodilator trial to assess reversibility.18
This study was approved by the Institutional Review Board (IRB) at the University of Michigan (HUM00095468). Written informed consent was given for the prospectively recruited controls. No informed consent was required for the retrospective HF cohort. Statistical analysis performed with STATA version 15.0 (Stata Corporation, College Station, Texas).
Results
Our study population consisted of 77 controls and 691 patients with heart failure (HF) (n = 768). Baseline demographic and clinical characteristics of the controls and HF patients are shown in table 2. VO2 and VO2 index are displayed in table 3 grouped by NYHA class and sex. Characteristics of patients who underwent right heart catheterization are presented in the online supplement Table 1. Of the HF patients, 48% (n = 330) were NYHA class IV at the time of measurement.
Table 2.
Baseline Characteristics at Time of Measured Oxygen Consumption Comparing Controls and NYHA class Values are reported as mean ± SD or n (%)
NYHA, New York Heart Association; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; NICM, non-ischemic cardiomyopathy; PVD, peripheral vascular disease; SD, standard deviation.
| Controls | NYHA class I | NYHA class II | NYHA class III | NYHA class IV | Total | |
|---|---|---|---|---|---|---|
| N=77 | N=17 | N=103 | N=241 | N=330 | N=768 | |
| Age (years) | 51.2 ± 15.2 | 41.9 ± 16.0 | 56.3 ± 12.9 | 58.2 ± 12.3 | 57.0 ± 13.5 | 56.4 ± 13.6 |
| Female | 51 (66.2%) | 5 (29.4%) | 25 (24.3%) | 82 (34.0%) | 108 (32.7%) | 271 (35.3%) |
| Height (inches) | 66.9 ± 3.8 | 67.6 ± 3.9 | 68.6 ± 4.0 | 68.1 ± 3.8 | 67.8 ± 4.0 | 67.9 ± 3.9 |
| Weight (kg) | 77.1 ± 15.9 | 88.9 ± 21.0 | 90.2 ± 24.1 | 90.3 ± 23.0 | 88.9 ± 25.3 | 88.3 ± 23.8 |
| BMI | 26.7 ± 4.8 | 30.1 ± 6.3 | 29.5 ± 6.7 | 30.1 ± 6.9 | 29.8 ± 7.8 | 29.6 ± 7.1 |
| BSA(m2) | 1.9 ± 0.2 | 2.0 ± 0.3 | 2.0 ± 0.3 | 2.0 ± 0.3 | 2.0 ± 0.3 | 2.0 ± 0.3 |
| VO2* | 223 ± 42 | 243 ± 50 | 231 ± 64 | 218 ± 55 | 203 ± 60 | 214 ± 58 |
| VO2index† | 117 ± 15 | 119 ± 21 | 110 ± 20 | 105 ± 20 | 99 ± 22 | 104 ± 21 |
| CAD | - | 4 (23.5%) | 35 (34.0%) | 128 (53.1%) | 176 (53.3%) | 343 (49.6%) |
| Diabetes Mellitus | - | 1 (5.9%) | 34 (33.0%) | 96 (39.8%) | 149 (45.2%) | 280 (40.5%) |
| COPD | - | 0 (0.0%) | 12 (11.7%) | 42 (17.4%) | 60 (18.2%) | 114 (16.5%) |
| Atrial Fibrillation | - | 5 (29.4%) | 40 (38.8%) | 110 (45.6%) | 166 (50.3%) | 321 (46.5%) |
| NICM | - | 10 (58.8%) | 67 (65.0%) | 105 (43.6%) | 158 (47.9%) | 340 (49.2%) |
| Hypertension | - | 5 (29.4%) | 48 (46.6%) | 129 (53.5%) | 154 (46.7%) | 336 (48.6%) |
| PVD | - | 1 (5.9%) | 5 (4.9%) | 25 (10.4%) | 31 (9.4%) | 62 (9.0%) |
VO2 (ml O2/min);
VO2 index (ml O2/min/m2)
Table 3.
Baseline oxygen consumption and oxygen consumption index grouped by NYHA class and se Values are reported as mean (SD).
NYHA, New York Heart Association; VO2, oxygen consumption.
| Control | NYHA class I | NYHA class II | NYHA class III | NYHA class IV | |
|---|---|---|---|---|---|
| Male | N=26 | N=12 | N=78 | N=159 | N=222 |
| VO2* | 260 (34) | 262 (48) | 241 (62) | 230 (53) | 217 (60) |
| VO2 index† | 125 (13) | 124 (23) | 111 (21) | 107 (19) | 102 (22) |
| Female | N=51 | N=5 | N=25 | N=82 | N=108 |
| VO2* | 204 (33) | 197 (16) | 200 (60) | 196 (52) | 174 (48) |
| VO2 index† | 113 (15) | 106 (2) | 107 (20) | 100 (20) | 92 (19) |
VO2 (ml O2/min);
VO2 index (ml O2/min/m2)
Univariable and multivariable linear regression analyses are presented in table 4. In the multivariable analysis adjusting for age, sex, and BSA, increasing NYHA class was associated with significantly lower VO2 in a stepwise fashion compared to controls (β NYHA class 4 vs controls = −36 ml O2/min, P < 0.001). The model explained over half of the observed variability in VO2 (r2 = 0.54). The variance inflation factor analysis did not show evidence of multicollinearity (mean 1.91, range 1.11 – 3.20) and the residual plot did not show heteroscedasticity. When body mass index is used in place of BSA, the results were similar with respect to NYHA class. Similarly, the inclusion of ejection fraction and resting hemodynamics (right atrial pressure and pulmonary capillary wedge pressure) in a model of HF patients did not change the associated relationship between VO2 and NYHA class. The predicted marginal mean effect of NYHA class on VO2 is shown in Figure 1.
Table 4.
Results of the univariable and multivariable regression analysis predicting resting oxygen consumption
CI, confidence interval; NYHA, New York Heart Association; BSA, Body surface area
| Predictor | Univariable | Multivariable | ||
|---|---|---|---|---|
| Regression coefficient | 95% CI | Regression coefficient | 95% CI | |
| NYHA Class (vs control) | ||||
| NYHA class I | 20 | −11 - 50 | −9 | −30 – 12 |
| NYHA class II | 8 | −9 - 25 | −14* | −26 - −2 |
| NYHA class III | −5 | −20 - 10 | −23‡ | −33 - −13 |
| NYHA class IV | −20† | −34 - −6 | −36‡ | −46 - −26 |
| Age (per 1 y) | −1.3‡ | −1.5 - −1.0 | −0.8‡ | −1.0 - −0.6 |
| Female (vs male) | −39‡ | −47 - −31 | −10† | −16.7 - −3.2 |
| BSA (per m2) | 144.4‡ | 133.3 – 155.6 | 134.6‡ | 122.8 - 146.5 |
p<0.05
p<0.01
p<0.001
Figure 1.
The posterior estimations of the marginal mean effect of NYHA class on VO2 showing predicted VO2 for each NYHA class. Controls labelled NYHA class 0.
NYHA, New York Heart Association.
Comparisons of measured and calculated VO2 for controls and patients are shown in the online supplement table 2. For male HF patients, the measured V02 was significantly overestimated by calculations with the exceptions of Dehmer and LaFarge estimations for NYHA class I patients. For female HF patients, the Dehmer, and Bergstra equations significantly overestimated the VO2 for all patients. The LaFarge equation significantly underestimated the VO2 for NYHA class II patients while overestimating the VO2 for NYHA class IV patients. There was no significant difference between the measured and calculated value for females with NYHA class I and III symptoms using the LaFarge equation. Td derived VO2 overestimated VO2 for all patients with the exception of female NYHA class I patients.
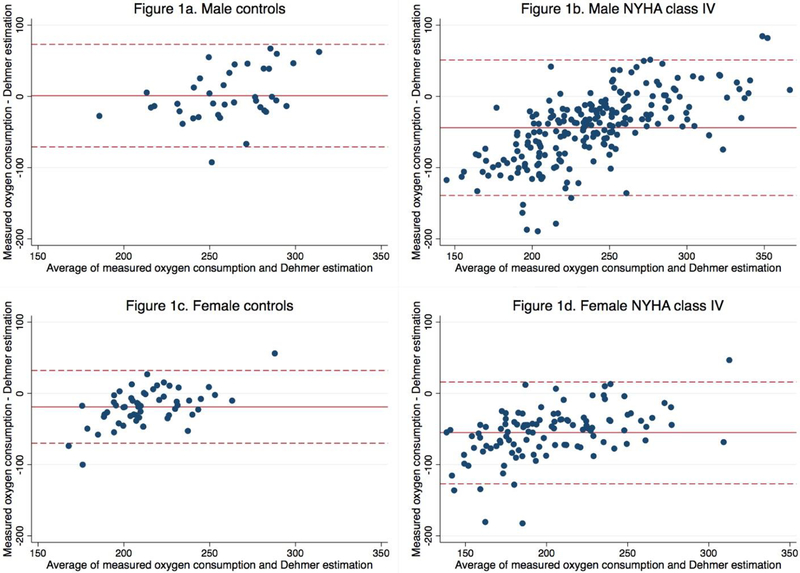
Bland-Altman plots for male and female controls and NYHA class IV patients illustrate a fixed bias towards overestimation when using the Dehmer equation in male and female patients (Figure 2). The mean bias for NYHA class IV males and females was −44 ml O2/min and −55 ml O2/min respectively. There are wide limits of agreement and overestimation at low VO2 and underestimation at high VO2 in NYHA class IV patients.
Figure 2.
Bland-Altman plots for male and female controls (1a, 1c) and patients (1b, 1d) with NYHA class IV symptoms comparing measured oxygen consumption (VO2) vs estimated oxygen consumption using the Dehmer formula. The results show wide limits of agreement and overestimation at low VO2 and underestimation at high VO2 NYHA class IV patients.
NYHA, New York Heart Association.
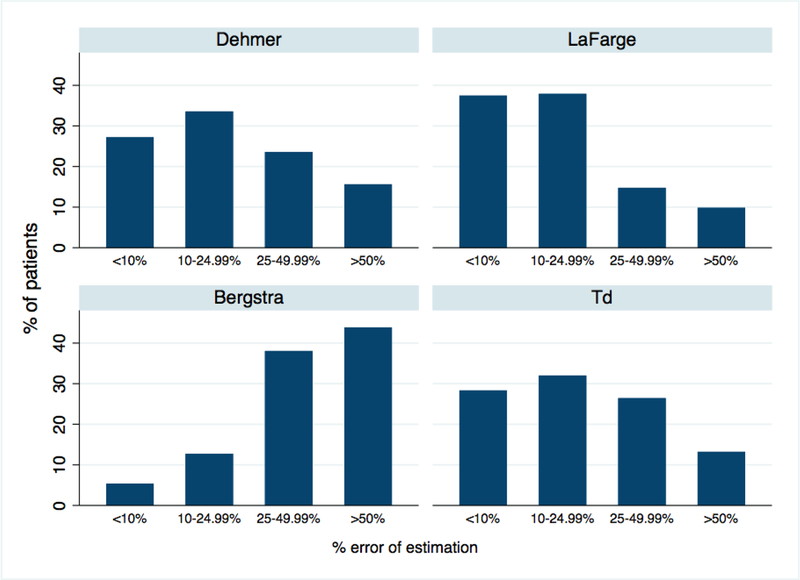
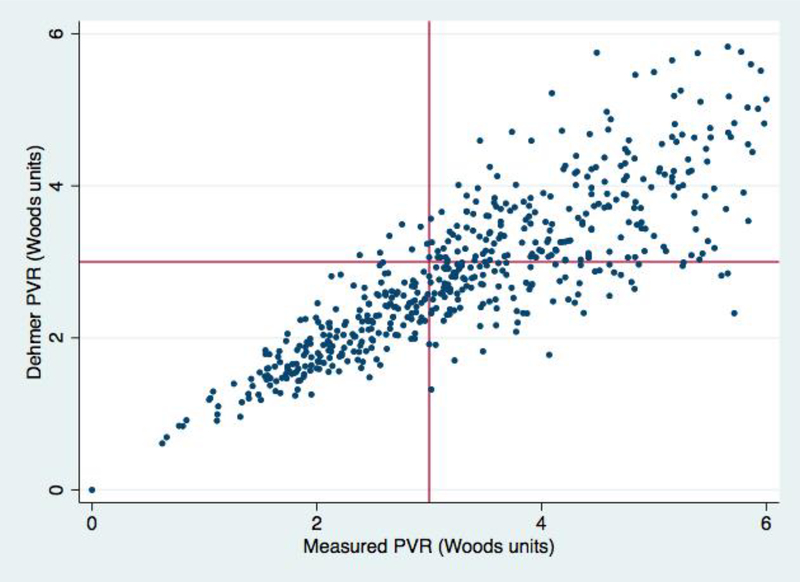
The mean percent differences of the measured VO2 and estimated VO2 using published equations were high in HF patients (n = 691) with 39% (n=271), 25% (n=170), 82% (n=566), and 39% (n=271) having a greater than a 25% difference using the Dehmer, LaFarge, Bergstra, and Td derived VO2, respectively (Figure 3). Of the 691 HF patients, 16% (n = 113), 33% (n = 227), and 4% (n = 29) would have had a PVR categorized as acceptable when the PVR calculated with the measured VO2 was >3 Woods units using the Dehmer, Bergstra, and LaFarge formulas respectively. The results for the Dehmer formula are shown in Figure 4.
Figure 3.
The percent difference in cardiac output using measured oxygen consumption compared to estimated oxygen comsumption in the previously published equations. There was a greater than 25% difference in 39% of patients using the Dehmer formula, 25% using the LaFarge formula, 82% using the Bergstra formula, and 39% using the Td derived VO2.
Td, thermodilution.
Figure 4.
Scatter plot comparing the calculated PVR from measured oxygen consumption vs estimated oxygen consumption using the Dehmer calculation. Of the HF patients, 16% (113/691) have a PVR of <3 Woods units using the Dehmer calculation while PVR >3 Woods units using the measured oxygen consumption (right lower quadrant). 1% (7/691) have a PVR of <3 Woods units using the measured oxygen consumption formula while PVR >3 Woods units using the Dehmer calculation (left upper quadrant).
PVR, pulmonary vascular resistance.
Discussion
Our study sought to compare the resting VO2 of controls and HF patients grouped by NYHA class, determine the accuracy of estimated VO2, and determine the potential clinical impact of errors in estimation. To our knowledge, this is the first study demonstrating the association between heart failure symptoms and resting VO2. We found that measured VO2 was reduced in HF patients with increasing NYHA class. In general, estimations are inaccurate and tend to overestimate VO2 leading to significant bias. Lastly, incorporation of estimated VO2 in our HF population could potentially miscategorize many patients as optimized for transplant, when the measured VO2 shows that they have a high PVR.
The inaccuracy in estimations of VO2 has been reported in a variety of patient populations.10–14 Previously, age, sex, BMI, HR, and low ejection fraction have all been associated with changes in resting VO2.7–9, 12–14 Given the small and relatively homogenous populations used to derive estimation equations,7–9 understanding the limitations of applying estimations to advanced HF patients is necessary. The only study that used ejection fraction as an inclusion criterion found that estimates were generally inaccurate.14 Thus, a more robust understanding of this in symptomatic patients with HF becomes critical. In our study, male control patients had a VO2 index of 125 (±13) ml O2/min/m2 (table 3), similar to the Dehmer formula for VO2.8 Also, while still inaccurate, estimations of VO2 performed better in controls and patients with NYHA functional class I symptoms. This may be related to similarities in the populations in which the predictive formulas were derived. Importantly, we found decreasing resting VO2 in association with increasing NYHA functional class and that estimations of VO2 generally overestimated VO2 in this patient population. Building upon the prior work by Chase et. al.,14 we found that measured VO2 was not associated with reduced EF while it was highly associated with NYHA functional class symptoms. Thus, while estimations may be inaccurate in participants with low EF, the ejection fraction does not account for these differences. These novel finding are critically important as symptomatic advanced HF patients are the group that would most likely undergo catheterization.
Our study does not provide insight to the mechanism for differences in resting VO2, but prior studies evaluating skeletal muscle histology and physiology may offer possible explanations. Investigators have compared skeletal muscle structure in heart failure patients to normals and have reported reduced mitochondrial volume,19, 20 reduced capillary density,21 and atrophy of skeletal myocytes.22 Additionally, others have demonstrated differences in skeletal muscle physiology, with increased vasoconstriction,23,24 changes in vascular stiffness,23, 24 and reduced nitric oxide bioavailability.25 These differences may contribute to reduced VO2 at rest.
The clinical implications of our findings are significant. Right heart catheterization is used to assess cardiac output and is an important component in the evaluation and management of acute and chronic heart failure, particularly in patients being considered for advanced therapies including transplant and mechanical circulatory support.18, 26 The UNOS guidelines for transplant listing incorporate cardiac index as part of the determination of transplant listing status for heart allocation.15 The inaccurate calculation of cardiac output has the potential to lead to misdiagnosis of cardiogenic shock and initiation of therapies including inotropes that have potential to cause harm and have been associated with both short-term and long-term adverse outcomes including an increase in mortality.27–32 Equally important, as we saw in our study, inaccurate determination of cardiac output could have significant downstream repercussions including potential miscategorization of PVR. In our patients, the Dehmer equation grossly overestimated (>25%) the cardiac output in 39% of our patients. This contributed to an underestimation of PVR and could lead to a miscategorization of patients as eligible for heart transplant listing without further vasodilator testing.18 Thus, our study adds to the body of evidence that cautions against the use of estimations of VO2 in patients with advanced HF and worsening symptoms, a population for which error could lead to improper management decisions.
One potential solution would be to exclusively use Td CO when making clinical decisions. A recent large retrospective cohort study found only a modest correlation between Fick CO using estimated VO2 and Td CO. In that study, Td CI better predicted mortality.33 Given the importance of CO in the clinical decision making and management of HF patients, the potential inaccuracy of Td CO introduced by TR and low CO, both of which are often present in end-stage heart failure, remains a concern.2–5 Additionally, it does not appear that using Td derived VO2 is preferential as it also overestimated VO2 in this HF population. A shift from using estimations of VO2 to measured VO2, particularly in severely symptomatic patients, represents an opportunity to improve patient care and better identify patients who may benefit from advanced therapies such as left ventricular assist device or heart transplantation. Further studies are necessary to determine whether cardiac output using a measured VO2 and the Fick method is superior to the Td technique.
Our study has limitations. We used the documented NYHA class closest to the time of VO2 measurement. As NYHA class is a fluid variable in individual patients, the NYHA class assigned to a patient at the time of a clinic visit may not have been the same assigned at the time of the right heart catheterization. Heart failure symptoms can vary significantly in a short period of time and there can be variability in interpretation of NYHA class.34 Additionally, there were only a small number of NYHA class I patients who underwent VO2 measurement. We did not use this group as a comparison for statistical analysis, therefore, it does not impact the primary findings of the study. In the study, multiple operators measured VO2. While a standardized protocol is used, differences in the individual methods could have influenced the variability of the measurement. We would suspect that this would bias the results to the null which we did not observe. Lastly, this is a single-center study of a tertiary-care center with a high volume of advanced heart failure patients. Whether our results can be extrapolated to the general population of heart failure patients is unknown.
Despite these limitations, given the clear association with decreasing VO2 with increasing NYHA class, the inaccuracy of measured to estimated VO2, and the potential clinical implications, measured rather than estimated VO2 should be used when calculating cardiac output with the Fick principle to guide therapies in patients with advanced HF.
Supplementary Material
Funding
This study was supported by the clinical research fellowship from the Heart Failure Society of America supported by Medtronic. Dr. Cascino was supported by a National Institutes of Health T32 postdoctoral research training grant (T32-HL007853).
Abbreviations:
- HF
Heart failure
- CO
cardiac output
- Td
thermodilution
- TR
tricuspid regurgitation
- VO2
oxygen consumption
- NYHA
New York Heart Association
- ROCA
resting oxygen consumption assessment
- BSA
body surface area
- PVR
pulmonary vascular resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Dr. Grafton: None. Dr. Cascino: None. Dr. Perry: None. Dr. Ashur: None. Dr. Koelling receives research support from scPharmaceuticals.
References
- 1.Fick A Uber die Messung des Blutquantums in Den Herzventrikeln. Sitzungsberichete der pysmed. Gesellschaftz zu Wurzburg. 1870;XVI–XVII. [Google Scholar]
- 2.van Grondelle A, Ditchey RV, Groves BM, Wagner WW Jr and Reeves JT. Thermodilution method overestimates low cardiac output in humans. The American journal of physiology. 1983;245:H690–2. [DOI] [PubMed] [Google Scholar]
- 3.Cigarroa RG, Lange RA, Williams RH, Bedotto JB and Hillis LD. Underestimation of cardiac output by thermodilution in patients with tricuspid regurgitation. The American journal of medicine. 1989;86:417–20. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa T and Dohi S. Errors in the measurement of cardiac output by thermodilution. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 1993;40:142–53. [DOI] [PubMed] [Google Scholar]
- 5.Hillis LD, Firth BG and Winniford MD. Analysis of factors affecting the variability of Fick versus indicator dilution measurements of cardiac output. Am J Cardiol. 1985;56:764–8. [DOI] [PubMed] [Google Scholar]
- 6.Fick A Ueber die messung des blutquantums in den herzventrikeln. Sitz der Physik-Med Ges Wurzburg. 1870;2:16–28. [Google Scholar]
- 7.LaFarge CG and Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. [DOI] [PubMed] [Google Scholar]
- 8.Dehmer GJ, Firth BG and Hillis LD. Oxygen consumption in adult patients during cardiac catheterization. Clin Cardiol. 1982;5:436–40. [DOI] [PubMed] [Google Scholar]
- 9.Bergstra A, van Dijk RB, Hillege HL, Lie KI and Mook GA. Assumed oxygen consumption based on calculation from dye dilution cardiac output: an improved formula. Eur Heart J. 1995;16:698–703. [DOI] [PubMed] [Google Scholar]
- 10.Kendrick AH, West J, Papouchado M and Rozkovec A. Direct Fick cardiac output: are assumed values of oxygen consumption acceptable? Eur Heart J. 1988;9:337–42. [DOI] [PubMed] [Google Scholar]
- 11.Wolf A, Pollman MJ, Trindade PT, Fowler MB and Alderman EL. Use of assumed versus measured oxygen consumption for the determination of cardiac output using the Fick principle. Cathet Cardiovasc Diagn. 1998;43:372–80. [DOI] [PubMed] [Google Scholar]
- 12.Narang N, Gore MO, Snell PG, Ayers CR, Lorenzo S, Carrick-Ranson G, Babb TG, Levine BD, Khera A, de Lemos JA and McGuire DK. Accuracy of estimating resting oxygen uptake and implications for hemodynamic assessment. Am J Cardiol. 2012;109:594–8. [DOI] [PubMed] [Google Scholar]
- 13.Narang N, Thibodeau JT, Levine BD, Gore MO, Ayers CR, Lange RA, Cigarroa JE, Turer AT, de Lemos JA and McGuire DK. Inaccuracy of estimated resting oxygen uptake in the clinical setting. Circulation. 2014;129:203–10. [DOI] [PubMed] [Google Scholar]
- 14.Chase PJ, Davis PG, Wideman L, Starnes JW, Schulz MR and Bensimhon DR. Comparison of Estimations Versus Measured Oxygen Consumption at Rest in Patients With Heart Failure and Reduced Ejection Fraction Who Underwent Right-Sided Heart Catheterization. Am J Cardiol. 2015;116:1724–30. [DOI] [PubMed] [Google Scholar]
- 15.Policy 6: Allocation of Hearts and Heart-Lungs 2018.
- 16.Du BD and Du BE. Clinical calorimetry: Tenth paper a formula to estimate the approximate surface area if height and weight be known. Archives of Internal Medicine. 1916;XVII:863–871. [Google Scholar]
- 17.Bland JM and Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 18.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A, Council ISfHLTIID, Council ISfHLTIPT and Council ISfHLTIHFaT. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 19.Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, Riede U, Schlierf G, Kubler W and Schuler G. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. Journal of the American College of Cardiology. 1995;25:1239–49. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan MJ, Higginbotham MB and Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation. 1988;78:506–15. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Poole DC and Musch TI. Effect of heart failure on muscle capillary geometry: implications for 02 exchange. Medicine and science in sports and exercise. 1998;30:1230–7. [DOI] [PubMed] [Google Scholar]
- 22.Springer J, Springer JI and Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC heart failure. 2017;4:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corra U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani G and Agostoni P. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part II. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2010;17:643–8. [DOI] [PubMed] [Google Scholar]
- 24.Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corra U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani GQ and Agostoni P. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2010;17:637–42. [DOI] [PubMed] [Google Scholar]
- 25.Kingwell BA. Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:1685–96. [DOI] [PubMed] [Google Scholar]
- 26.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J and Transplantation ISfHaL. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–87. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor CM, Gattis WA, Uretsky BF, Adams KF, McNulty SE, Grossman SH, McKenna WJ, Zannad F, Swedberg K, Gheorghiade M and Califf RM. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999;138:78–86. [DOI] [PubMed] [Google Scholar]
- 28.Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E and Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2:320–4. [DOI] [PubMed] [Google Scholar]
- 29.Brozena SC, Twomey C, Goldberg LR, Desai SS, Drachman B, Kao A, Popjes E, Zimmer R and Jessup M. A prospective study of continuous intravenous milrinone therapy for status IB patients awaiting heart transplant at home. J Heart Lung Transplant. 2004;23:1082–6. [DOI] [PubMed] [Google Scholar]
- 30.Cuffe MS, Califf RM, Adams KF, Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M and Investigators OoaPToIMfEoCHFO-C. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–7. [DOI] [PubMed] [Google Scholar]
- 31.Elkayam U, Tasissa G, Binanay C, Stevenson LW, Gheorghiade M, Warnica JW, Young JB, Rayburn BK, Rogers JG, DeMarco T and Leier CV. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98–104. [DOI] [PubMed] [Google Scholar]
- 32.Hershberger RE, Nauman D, Walker TL, Dutton D and Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;9:180–7. [DOI] [PubMed] [Google Scholar]
- 33.Opotowsky AR, Hess E, Maron BA, Brittain EL, Baron AE, Maddox TM, Alshawabkeh LI, Wertheim BM, Xu M, Assad TR, Rich JD, Choudhary G and Tedford RJ. Thermodilution vs Estimated Fick Cardiac Output Measurement in Clinical Practice: An Analysis of Mortality From the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) Program and Vanderbilt University. JAMA cardiology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, Mayet J and Francis DP. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen-Solal A, Jacobson AF and Pina IL. Beta blocker dose and markers of sympathetic activation in heart failure patients: interrelationships and prognostic significance. ESC heart failure. 2017;4:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.