Figure 1. The classical model for regulation of the G1/S transition by cyclins and CDKs.
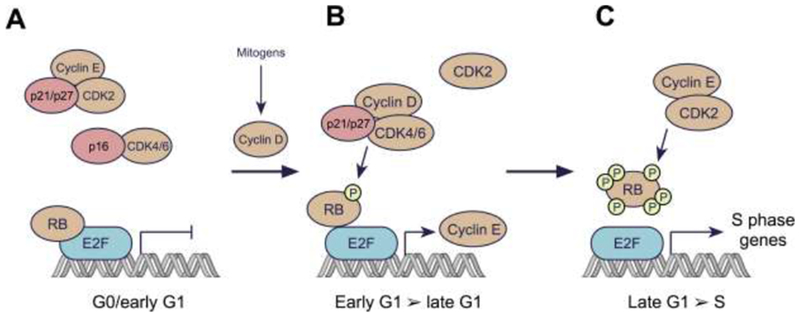
A: In resting cells, CDK4/6 and CDK2 are inactive. D-type cyclin levels are low due to the lack of mitogenic stimulus, limiting CDK4/6 activity. Moreover, CDKs 4 and 6 are bound by INK4 family members (e.g. p16), establishing binary complexes that lack kinase activity. CDK2 complexes are inhibited by the CIP/KIP proteins p21 and p27. Collectively, the suppression of CDK4/6 and CDK2 leads to RB hypo-phosphorylation, and hence repressed expression of E2F target genes. This repression is mediated by direct blockade of the E2F transactivation domain by RB, and by recruitment of histone modifiers to RB that further silence E2F target gene expression.
B: Levels of D-type cyclins increase in response to mitogenic stimuli, due to both enhancement of cyclin D gene expression and an increase in cyclin D protein stability. D-type cyclins bind to CDK4/6, forming complexes that are stabilized by p21 or p27. Cyclin D:CDK4/6 complexes then enter the nucleus and phosphorylate RB. This partially de-represses expression of E2F target genes, including those for the E-type cyclins. The partial phosphorylation of RB facilitates progression through G1.
C: As the levels of E-type cyclins rise in late G1, CDK2 is activated resulting in RB hyperphosphorylation and inactivation. Hyperphosphorylated RB is released from E2F, enabling increased transcription of E2F target genes necessary for the cell to proceed into S phase.
