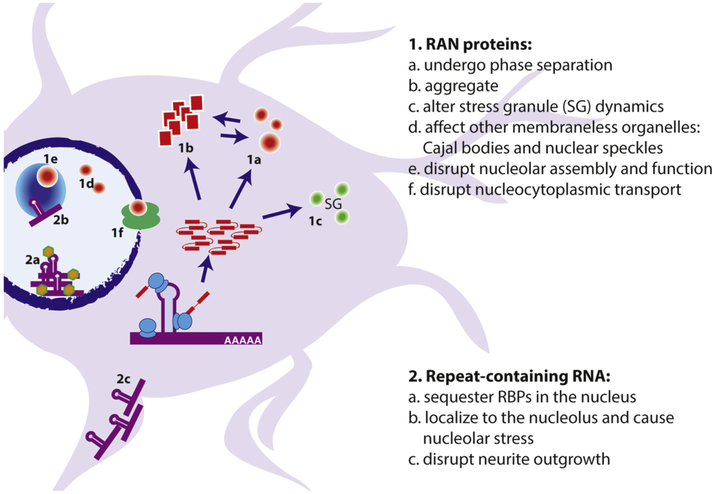
Figure 4: Mechanisms by which transcribed repeats elicit neurodegeneration.
Expanded repeats can be toxic as RNA or as protein. 1) poly(GR) and poly(PR) containing RAN DPR proteins undergo phase separation (1a) into liquid-like droplets (LLPS) in vitro and in vivo. Poly(GA), poly(GR), and poly(PR) RAN proteins can also alter stress granule assembly and dynamics (1b) and form insoluble aggregates (1c). In the nucleus, RAN proteins can impair the dynamics of Cajal bodies and membraneless organelles (1d), and disrupt nucleolar function and ribosomal biogenesis (1e). They can also disrupt the nuclear envelope and nuclear pore complex (1f). Repeat containing RNAs can sequester important RNA-binding proteins in the nucleus and can themselves undergo LLPS, perhaps in concert with RBPs (2a). Repeat RNAs that exit the nucleus can be exported to neuronal processes where they can impair neurite outgrowth and potentially alter mRNP dynamics (2b).