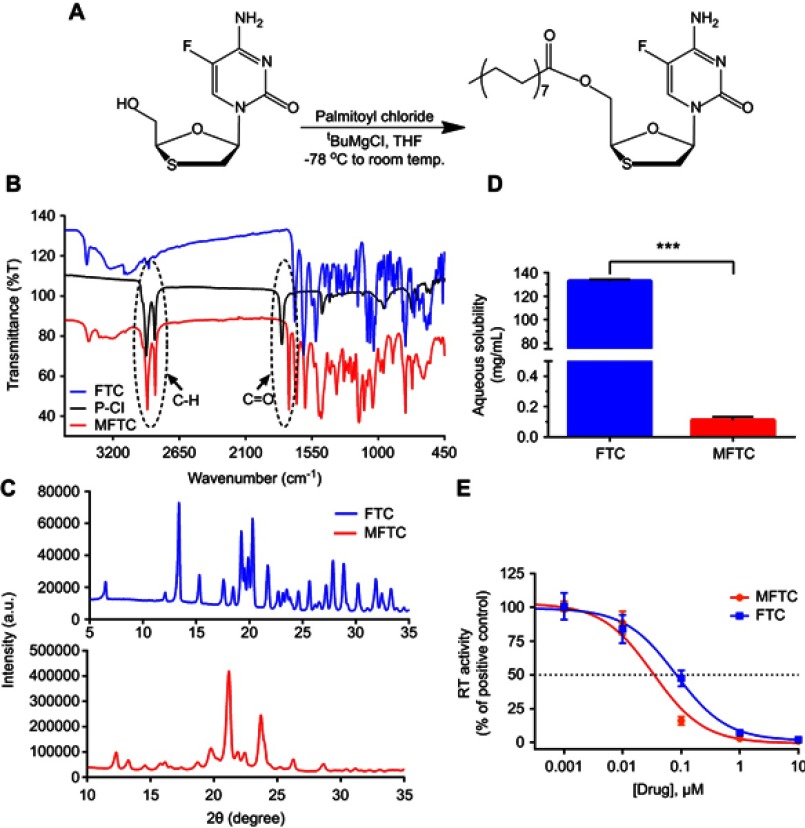
Figure 1.
Synthesis and characterization of MFTC. (A) A 16-carbon fatty acid modified MFTC was synthesized with a final yield of 90%. (B) FTIR spectrum of MFTC showing, highlighted in dashed circles, absorption peaks at 2920 and 2860 cm−1 that correspond to CH2-CH2 stretches of the fatty acid chain and at 1745 cm−1 that corresponds to the carbonyl functional group that is part of the formed ester bond. (C) XRD analysis of FTC and MFTC demonstrates the crystalline nature of both drugs. (D) Aqueous solubility of FTC and MFTC demonstrates decrease in solubility of MFTC (mean±SD, n=3; ***P≤0.001). (E) EC50 (dashed line) was determined in vitro by HIV-1 RT activity assay (0.079 and 0.033 µM for FTC and MFTC, respectively) demonstrating the chemical modification did not significantly change the antiretroviral activity of FTC. Results were analyzed by nonlinear regression fit (mean±SD, n=3).
Abbreviations: FTC, emtricitabine; MFTC, modified FTC prodrug; FTIR, Fourier transform infrared spectroscopy; XRD, X-ray diffraction; RT, reverse transcriptase; EC50, half maximum effective concentration.