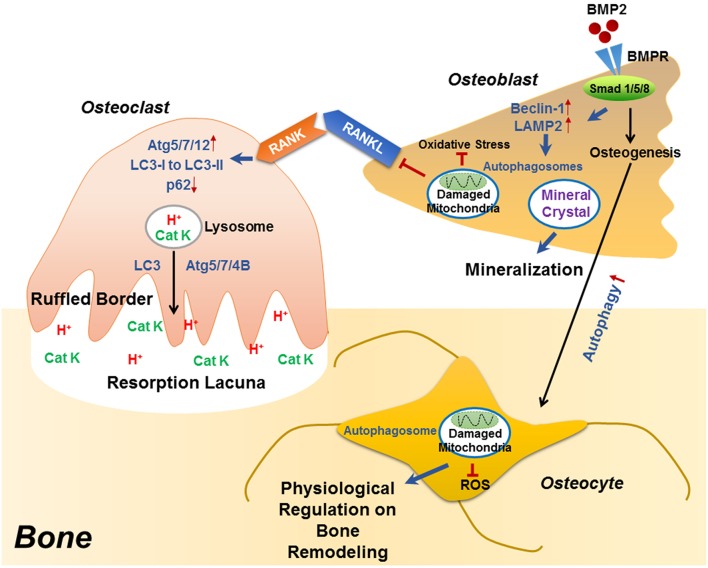
Figure 1.
The role of autophagy in the differentiation/function of osteoclast, osteoblast and osteocyte. During RANKL-RANK induced osteoclast differentiation, the protein levels of ATG5/7/12 are increased, accompanied with enhanced conversion from LC3-I to LC3-II and p62 degradation, which plays an essential role in the generation of F-actin ring. Besides differentiation, autophagy also plays decisive roles in osteoclast function, that the physiological levels of Atg5/7/4B are required for lysosomal [containing H+ and cathepsin K (CatK)] trafficking and fusion with the plasma membrane to generate mature ruffled border, as well as to release H+ and cathepsin K to resorb bone. During osteoblast differentiation, the binding of BMP2 to its receptors (BMPR) activates Smad signaling pathway to initiate osteogenesis, which also induced the expression of beclin 1 and LAMP2 (autophagy-related proteins) as well as autophagy pathway. Autophagosomes are utilized for transporting mineral crystals to extracellular matrix and thereby facilitating mineralization. Autophagy reduces oxidative stress during osteoblast differentiation via clearance of damaged mitochondria, which also suppresses RANKL production and hence inhibiting osteoclastogenesis. Compared with osteoblast, the autophagy level is increased in osteocyte, which not only maintains homeostasis of osteocyte, but also guarantees a physiological osteocyte-derived regulation on bone remodeling.