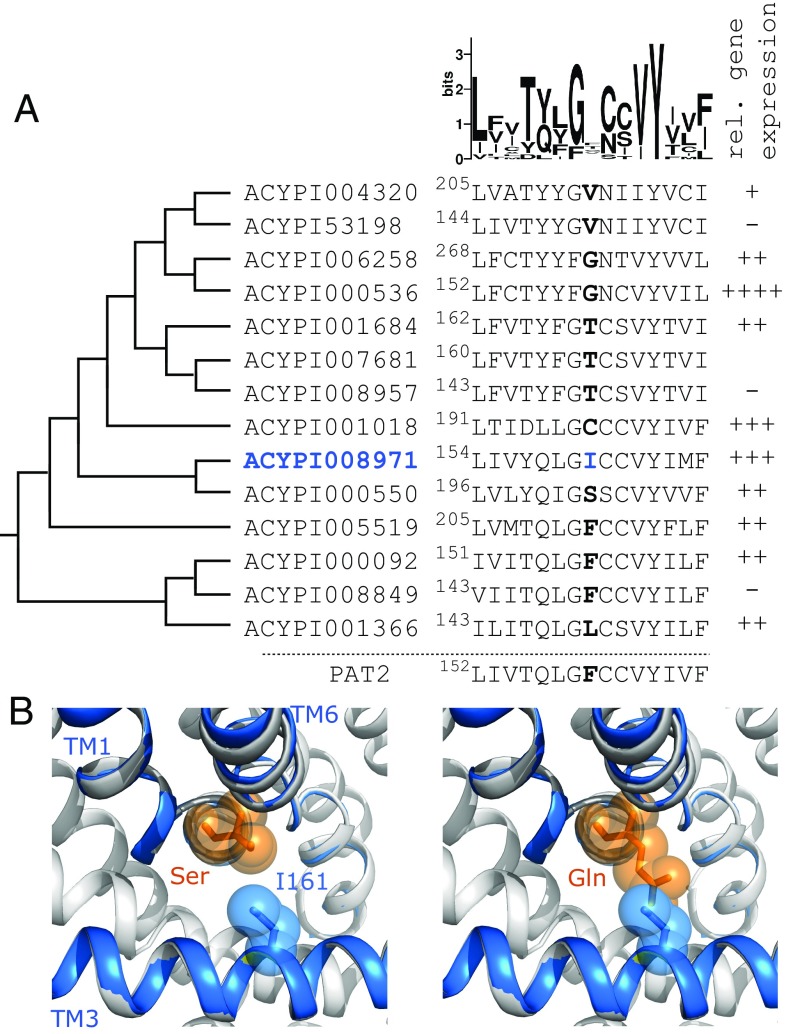
Fig. 2.
Identification of aphid ApNEAAT1 (ACYPI008971) as a putative carrier of small NEAAs expressed in the bacteriocyte. (A) Left column: phylogeny showing the relationship between all 14 SLC36-related A. pisum AAAP transporters from the arthropod expanded AAAP clade (30, 31). Phylogenetic tree based on previously published phylogenies (30, 50). Middle column: portion of a full sequence alignment (by PROMALS3D) showing the central section of TM3 with a representation of the variability at each residue position shown above as a Sequence Logo. The residues highlighted in bold are equivalent to both F159 in rat PAT2 (slc36a2) and V104 in LeuT (39, 51). ACYPI008971 has I161 (blue) at this residue position. Right column: Representation of relative gene expression of each transporter within the bacteriocyte structure as a whole. −, not expressed; ++++, most highly expressed; +++, ≤35%; ++, ≤15%, +, ≤1% expression of the most highly expressed amino acid transporter [summary of gene expression determined by RNAseq which is consistent with earlier estimates using qPCR (28, 30)]. ACYPI007681 expression was not determined. (B) A structural model of ACYPI008971 was created using I-TASSER and aligned against the highest-scoring crystal (3L1L of the arginine transporter AdiC, gray). Sections of ACYPI008971 TM1, TM3, and TM6 are shown as blue ribbons. ACYPI008971 I161 (blue sticks and spheres) projects toward the substrate binding pocket. When serine or glutamine (orange sticks and spheres) were positioned in the binding pocket, using the arginine in the 3L1L crystal as a guide, it shows that I161 is likely to limit binding pocket space so that ACYPI008971 may transport amino acids with shorter (Ser) rather than longer (Gln) side-chains.