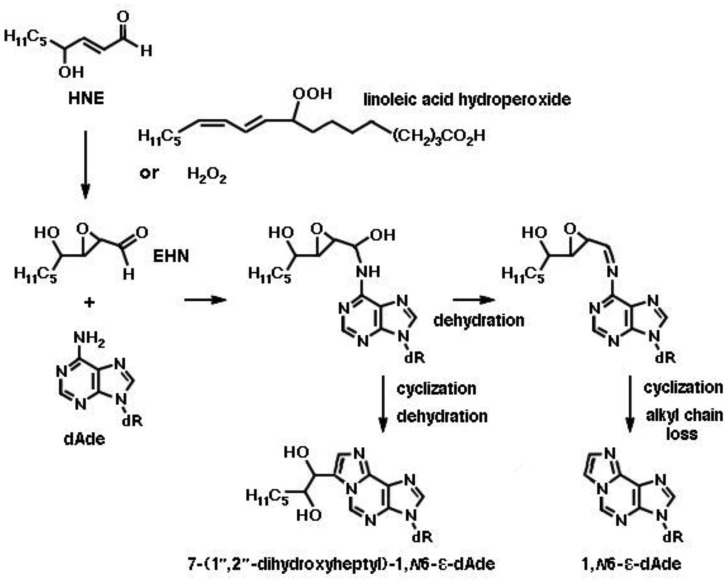
Figure 7. Exocyclic etheno adducts formed by the reaction of 2,3-epoxy-4-hydroxynonanal (epoxy-HNE, EHN) with deoxyadenosine (dAde) in DNA.
EHN is formed from HNE by incubation in the presence of a fatty acid hydroperoxide (linoleic acid hydroperoxide in the example) or hydrogen peroxide at 37 °C for 24 h [65]. Upon addition of EHN at the exocyclic N6 amino group of dAde, an intermediate is formed, which may either undergo cyclization by ring closure at position N1 and dehydration, yielding 7-(1′,2′-dihydroxyheptyl)-1,N6-etheno-deoxyadenosine (7-(1′,2′-dihydroxyheptyl)-1,N6-ε-dAde), or dehydration into an imine, followed by cyclization and loss of the alkyl side chain by a retroaldol reaction, yielding 1,N6-etheno-deoxyadenosine (1,N6-ε-dAde).