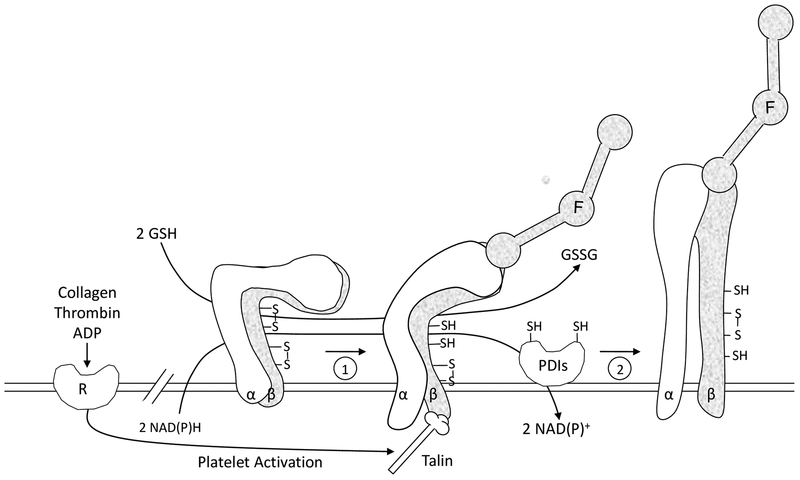
FIGURE 4.
Working model of the role of sulfhydryls and PDI in platelet function. The platelet fibrinogen receptor αIIbβ3 (α, β) is shown in three different activation states. The nonactivated state is on the left side. Redox sensitive disulfide bonds in αIIbβ3 are depicted. Agonist induced stimulation leads to cytoplasmic events and talin binding resulting in inside-out signaling and an initial ligand binding interaction of fibrinogen (F) with the receptor (1). A PDI catalyzed event then converts αIIbβ3 to the high affinity conformation (2) represented by secondary platelet aggregation. During platelet activation, sulfhydryls are generated in αIIbβ3 as well as in the active site of PDI from cytoplasmic reducing equivalents supplied by NAD(P)H. GSH or other low molecular weight thiols in the external redox environment also generate sulfhydryls in both αIIbβ3 and PDI facilitating the reactions shown.