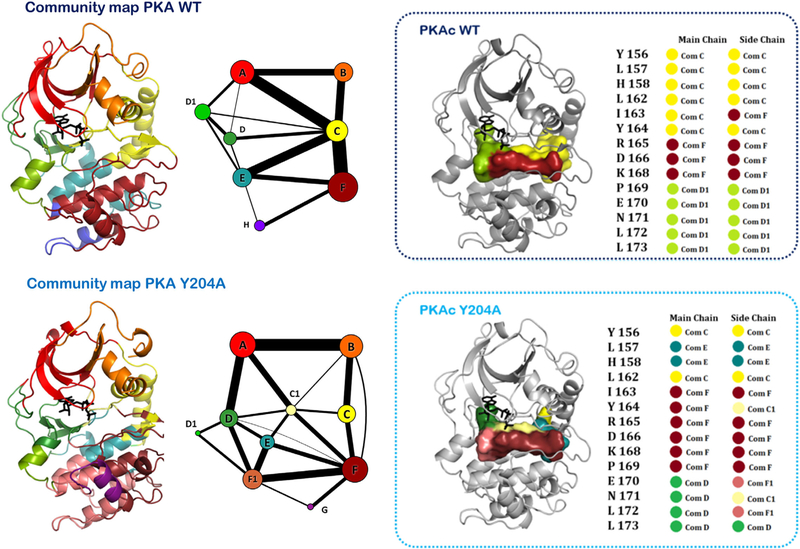
FIG 8.
Community map of PKA explored in the context of a distal Y204A mutation. The mutation about 8 Å away from the active site debilitates the kinetics of the kinase. Comparison of the community maps of the mutant and the wild-type PKA allow for understanding the dynamic allostery-based modulations that effect kinase activity. As shown in the active-site cleft, the distal Y204A mutation alters the distribution of the dynamics of the protein and reorganizes its community map. As a result, the mutant is unable to synchronize the nucleotide and peptide substrates optimally at the active site.