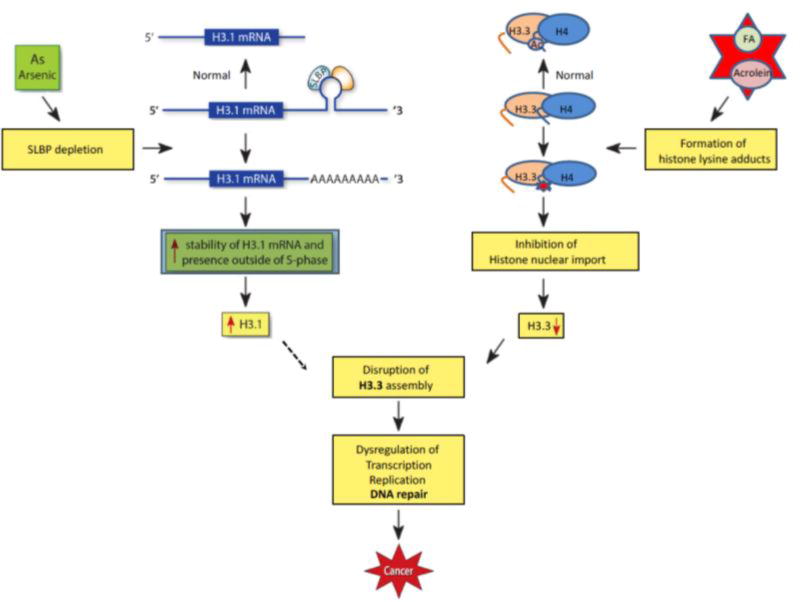
Figure 1. Disruption of chromatin structure by environmental exposures.
In normal condition, the stem-loop binding protein (SLBP) binds to the stem-loop structure on 3’ end of canonical histone H3.1 mRNA, generating the histone mRNA without poly(A) tail by cleaving downstream of the stem-loop. SLBP is depleted following arsenic exposure. The loss of SLBP results in binding of less SLBP to the stem-loop structure, adding a poly(A) tail via a downstream poly(A) signal sequence. The polyadenylation increases stability of H3.1 mRNA and subsequently H3.1 protein levels outside of S-phase, which may interfere with assembly of histone variant H3.3. On the other hand, aldehyde chemicals such as acrolein and formaldehyde (FA) form adducts with lysine residues on newly synthesized histones, including H3.3 and H4, inhibiting their acetylation and subsequently compromising histone nuclear import and assembly into chromatin. Defective chromatin assembly due to H3.3 reduction may dysregulate gene expression, DNA replication and repair thereby facilitating carcinogenesis.