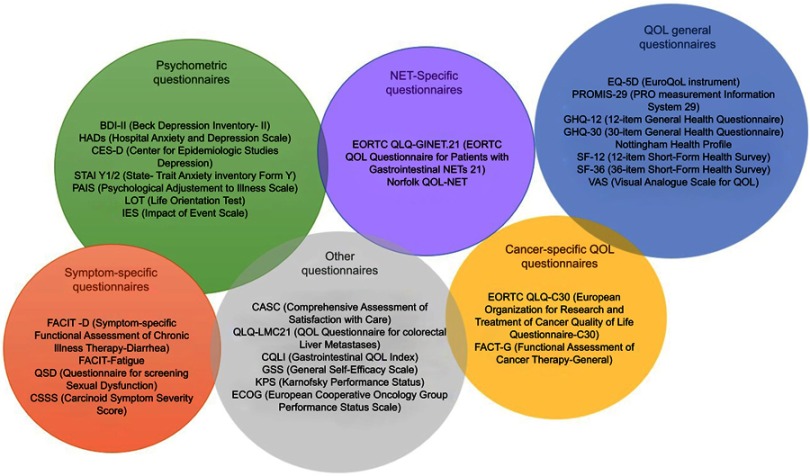
Figure 4.
Symptomatic assessment tools. The figure collects the different tools employed to evaluated quality of life in studies and trials of neuroendocrine tumors.
Abbreviations: BDI-II, Beck Depression Inventory-II; HADs, Hospital Anxiety and Depression Scale; CES-D, Center for Epidemiologic Studies Depression; STAI Y1/2, State-Trait Anxiety Inventory Fonn Y; PAIS, Psychological Adjustment to Illness Scale; LOT, Life Orientation Test; iES, Impact of Event Scale; FACIT-D, Functional Assessment of Chronic Illness Therapy-Diarrhea; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; QSD, Questionnaire for screening Sexual Dysfunction; CSSS, Carcinoid Symptom Severity Score; EORTC QLQ-GINET.21, European Organisation for Research and Treatment of Cancer Quality of life Questionnaire – Gastrointestinal Neuroendocrine Tumors; Norfolk QLQ-NET, Norfolk Quality of life Questionnaire for Neuroendocrine Tumors; EQ-5D, Euro-Quality of Life instrument; PROMIS-29, PRO measurement information system; GHQ-12, 12-item General Health Questionnaire; GHQ-30, 30-item General Health Questionnaire; SF-12, 12-item Short-Form Health Survey. SF-36: 36-item Short-Fonn Health Survey; VAS, Visual Analogue Scale for Quality of Life; EORTC QLO-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30; FACT-G, Functional Assessment of Cancer Therapy-General.