Figure 2.
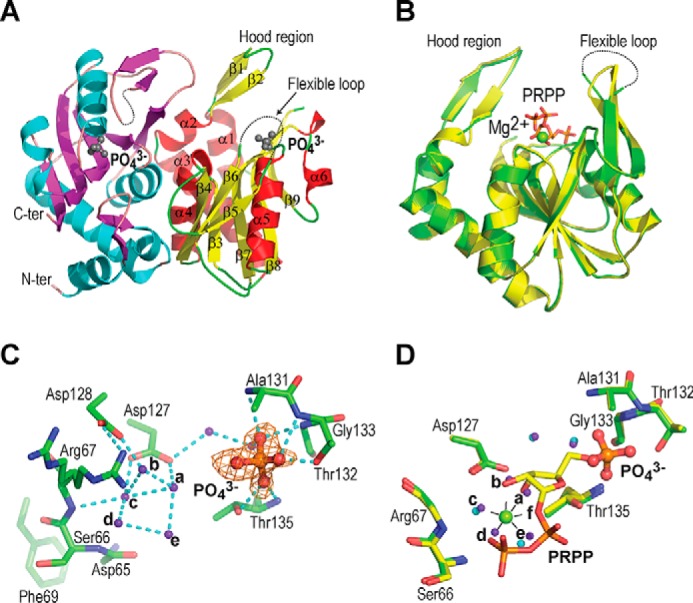
Structure of phosphate-bound hAPRT. A, structure of the hAPRT homodimer showing two phosphate molecules (gray ball-stick), the hood region, and the flexible loop. B, superimposition of the phosphate-bound (green) onto the PRPP-bound (yellow) hAPRT structure (RMSD = 0.20 Å). C, Fo – Fc omit electron density map for a phosphate ion contoured at 3σ. Without substrate bound in the active site, the conserved Arg-67 adopted two conformations. D, superimposition of the phosphate-bound (green) onto the PRPP-bound (yellow) hAPRT active site. Water molecules are shown in purple or cyan in the phosphate- or PRPP-bound structures, respectively. All the figures were generated with PyMOL. The hydrogen bonds are represented with dashed cyan lines throughout.
