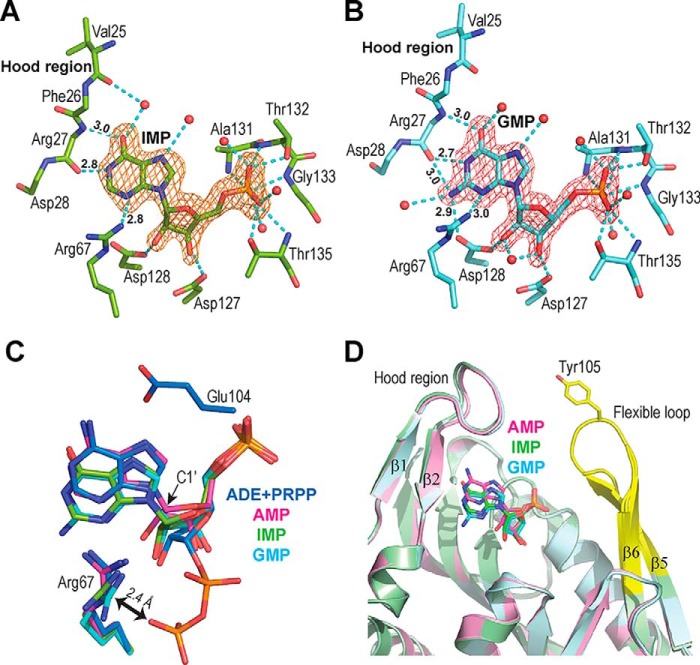
Figure 4.
Structure of two substrate analogs of the reverse reaction. A and B, Fo – Fc omit electron density maps for IMP (A) and GMP (B), contoured at 3σ from the IMP and GMP-hAPRT structures, respectively. Nonequivalent hydrogen bonds, as compared with the AMP-hAPRT structures, are indicated by their length in Angstroms. C, close-up view of the ligands after superimposition of the ADE-PRPP- (PDB ID: 6FCI), AMP- (PDB ID: 6FCL), IMP- and GMP-hAPRT structures. ADE and PRPP are in blue, AMP in magenta, IMP in green and GMP in cyan, with the corresponding amino acids in the active site colored accordingly. D, Superimposition of the AMP-, IMP-, and GMP-hAPRT structures showing the hood region and the flexible loop where the conserved Tyr-105 is located.