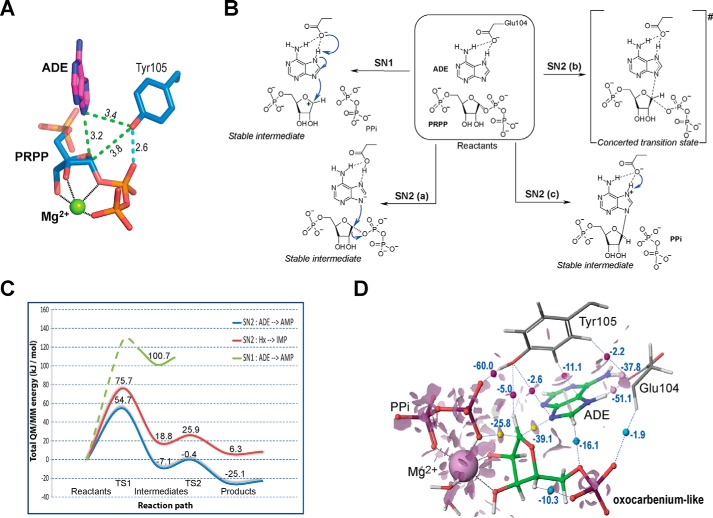
Figure 5.
Possible reaction paths for hAPRT. A, close contacts (green) and hydrogen bond (cyan) between ADE, PRPP, and Tyr-105 oxygen atom in the ADE-PRPP-Mg2+-hAPRT structure. B, proposed catalytic SN1 or SN2 pathways when hAPRT is complexed to ADE and PRPP. C, schematic representation of the reaction path for the forward reaction catalyzed by hAPRT. In green, the path found for the SN1 displacement mechanism involving ADE (only the stable intermediate was identified). The SN2 paths are shown in blue for ADE and in red for Hx. TS, transition state. D, visualization of the reduced density gradient surface. The electron density at a critical point between atoms, when the gradient of the density is null, provides information about the strength of a noncovalent interaction whereas the sign of the second derivative of the electron density distinguishes between bonded (negative value) and nonbonded (positive value) interactions. Consequently, at each critical point of a molecular system, an NCI strength coefficient is defined by multiplying the sign of the second derivative by the value of the electron density at this point. Hence, large negative values of this NCI strength coefficient are indicative of attractive interactions (dipole-dipole, hydrogen bonds, pi-pi stacking), large and positive values indicate that the interaction is nonbonding, whereas values near zero indicate weak van der Waals interactions. Critical points are indicated by yellow, pink, magenta, and cyan spheres with the associated NCI coefficient.