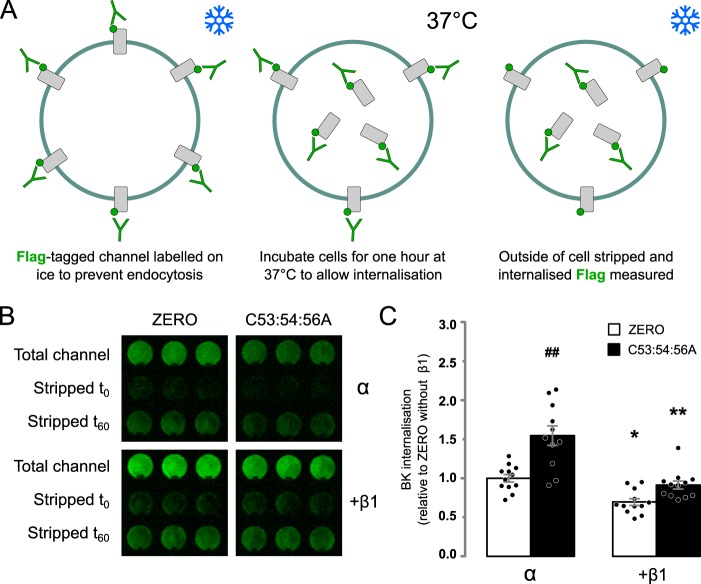
Figure 2.
β1-Subunit retards internalization of α-subunit. A, schematic outlining strategy for internalization assay in HEK293 cells. B, representative internalization assay of epitope-tagged BK α-subunit (ZERO) or C53:54:56A alone or co-expressed with WT β1-subunit in HEK293 cells. Internalized BK α-subunit was quantified, after 60 min at 37 °C, using the extracellular FLAG tag (green) following acid strip of surface staining in nonpermeabilized cells and normalized to surface expression at time 0. Three replicates from an individual experiment are shown. C, quantification of BK channel α-subunit internalization relative to the WT α-subunit internalization alone. There was a significant effect of α-subunit S-acylation and the presence of β1 subunits (H(3,48) = 26.85; p < 0.01, Kruskal–Wallis test). All data are means ± S.E. (n = 12). Kruskal–Wallis test with Dunn's post hoc analysis was used. *, p < 0.05; **, p < 0.01 compared with control (α-subunit alone). ##, p < 0.01 compared with WT (ZERO).