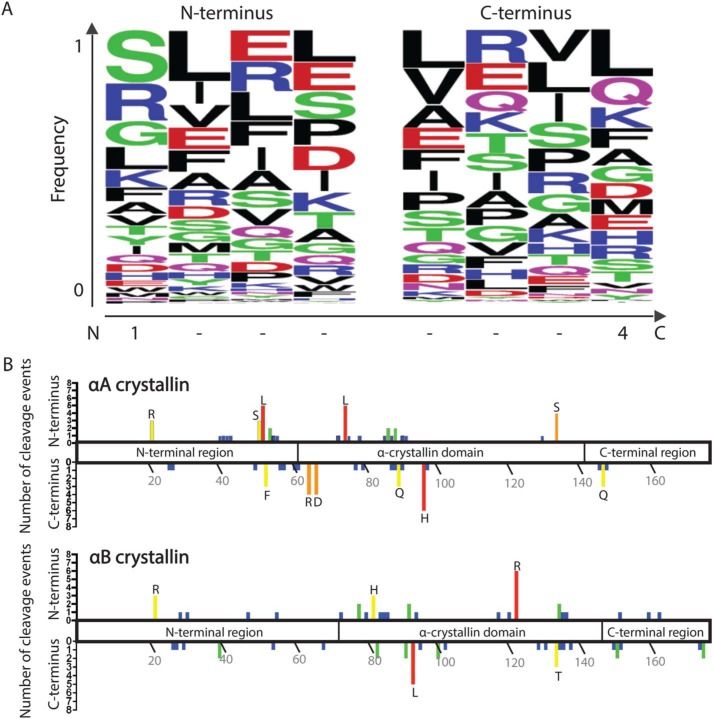
Figure 6.
The identified WS-HMW protein fragments do not display distinctive patterns at N or C termini, nor a strong localization of cleavage sites. A, the four amino acid residues present at the N- and C-terminal sequences of all peptides identified by MS in the WS-HMW fraction were inputted into WebLogo. The y axis represents the fraction of a particular residue that occurs at the indicated position. On the x axis, position 1 of the N terminus represents the first residue of the cleaved peptide, whereas position 4 of the C terminus represents the last residue of the cleaved peptide. B, graphical representation of the cleavage sites observed in WS-HMW peptides along the domains of αA crystallin (top) and αB crystallin (bottom). The upper y axis represents the number of times the residue indicated on the x axis was found at the N terminus of peptides. The lower y axis represents the number of times the residue indicated on the x axis was found at the C terminus of peptides. Residues with three or more cleavages are designated by their single-letter amino acid code.