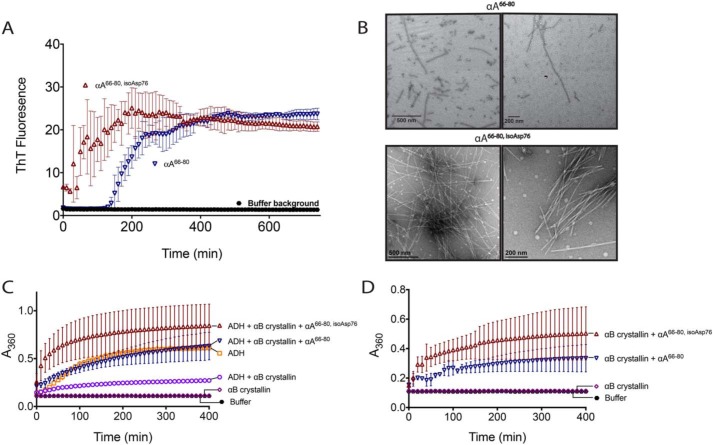
Figure 9.
Isomerization of the anti-chaperone peptide αA66–80 increases its ability to form amyloid and enhances its anti-chaperone activity in vitro. A, αA66–80 (▿) and αA66–80,isoAsp76 (▵) peptides were incubated with amyloid-binding dye thioflavin T to monitor self-aggregation as described under “Experimental Procedures.” B, peptides were incubated without ThT, and the resulting aggregates were observed by EM as described under “Experimental procedures.” Scale bar, either 200 or 500 nm, as indicated. Two representative fields are shown. C, aggregation was measured for ADH (150 μg) incubated under denaturing conditions (50 mm sodium phosphate, pH 7.0, 100 mm NaCl, and 10 mm phenanthroline) with and without αB crystallin (40 μg) and peptides (40 μg). Aggregation was monitored by light scattering at A360 nm. D, control aggregation reactions were performed as in C with peptides (40 μg) and αB crystallin (40 μg) alone. Data sets from C and D were from a single experiment and were separated onto two graphs for clarity; the αB crystallin alone and buffer background from C and D are duplicated. Error bars, S.D. of three replicates.