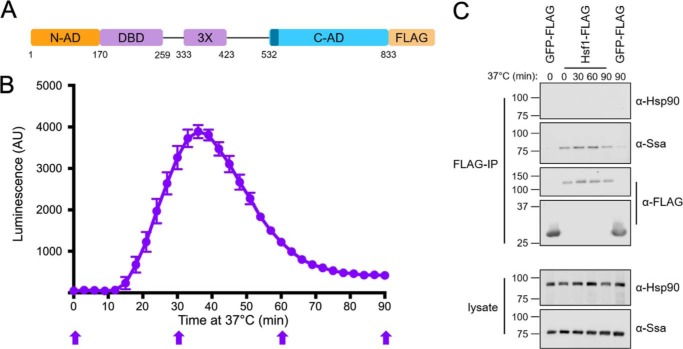
Figure 1.
The yeast Hsp70, but not Hsp90, interacts with Hsf1 during heat shock and attenuation. A, schematic of the yeast Hsf1 domain structure with C-terminal FLAG tag used for immunoprecipitation (IP). 3X, leucine zipper trimerization domain. CE2 is shown in dark blue. B, real-time luciferase reporter assay of Hsf1 activity over a 90-min heat shock at 37 °C. Error bars, S.D. of three biological replicates. C, Ssa1, but not Hsp90, co-immunoprecipitates with Hsf1-FLAG at the time points indicated in B with block arrows. GFP-FLAG is used as a negative control for nonspecific Ssa1 interaction. Immunoblots are representative of three independent experiments. AU, arbitrary units.