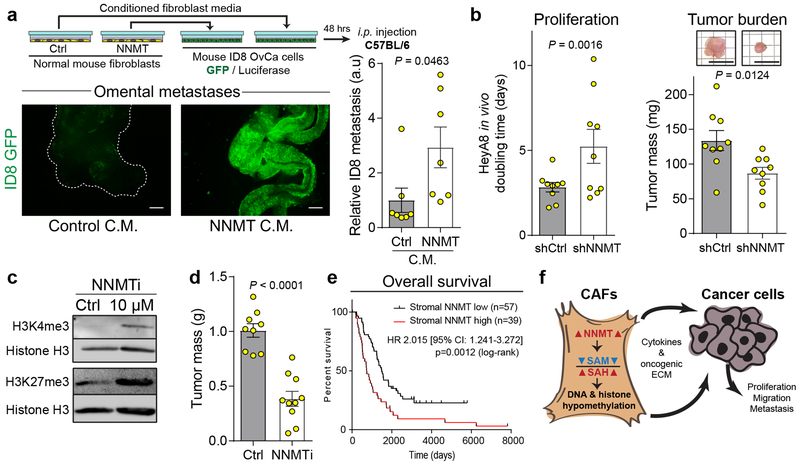
Fig. 4: Stromal NNMT supports HGSC progression and is associated with a poor prognosis.
(a) Schematic of experimental design (top). Representative images and quantification of omental adhesion following intraperitoneal injection of luciferase/GFP-labeled ID8 mouse OvCa cells treated with conditioned media from fibroblasts expressing the indicated constructs, n = 7 mice per group. Two-sided t-test. Scale bar = 500 μm. (b) In vivo proliferation and total tumor burden of luciferase-labeled OvCa cells co-injected with CAFs expressing shCtrl or shNNMT constructs, n = 9 tumors per group. Scale = 1 cm. (c) Treatment of CAFs with the NNMTi led to increased histone methylation. (d) Tumor burden of nude mice intraperitoneally injected with HeyA8 OvCa cells after 10 days of treatment with vehicle control (Ctrl; PBS; n = 9) or NNMTi (n = 10). Two-sided t-test. (e) Kaplan-Meier survival curves for patients with low (black) or high (red) stromal expression of NNMT in ovarian sites, as assessed by IHC. Two-tailed test. (f) Stromal NNMT drives ovarian cancer progression by metabolic regulation of histone methylation which causes epigenetic and transcriptional changes in stromal cells promoting cancer cell proliferation, migration, and metastasis. All bar graphs represent mean of data and error bars are SEM.