Figure7. GDC0941 and Vincristine Combinatorial Treatment in Preclinical Trials.
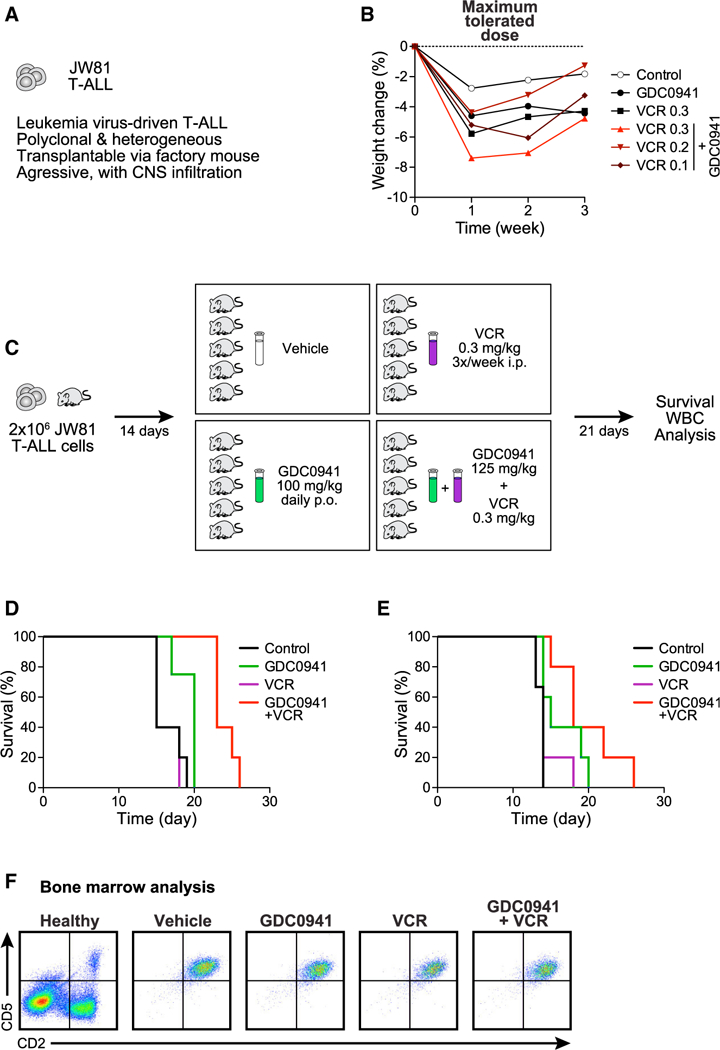
(A) Characteristics of murine T-ALL JW81.
(B) Body weight measurements over time to determine the maximum tolerated dose of GDC0941 (100 mg/kg body weight) and vincristine (VCR) (0.3 mg/kg body weight) alone, or for GDC0941 in combination with 3 different doses of VCR (mg/kg body weight).
(C) Dosing and application scheme for GDC0941 and VCR in preclinical trials.
(D) Survival curves for mice treated with vehicle control, GDC0941 alone, VCR alone, or the GDC0941/VCR combination therapy. GDC0941 at 125 mg/kg/day starting 4 days post-transplant and VCR at 0.3 mg/kg twice weekly starting 6 days post-transplant. Mantel-Cox tests comparing treatments provided the following p values: control versus VCR p = 0.6015, control versus GDC0941 p = 0.0323, VCR versus GDC0941 p = 0.0392, VCR versus combination p = 0.0023, GDC0941 versus combination p = 0.0050, and control versus combination p = 0.0015. For additional statistical analysis, see Figure S6.
(E) As in (D), but GDC0941 at 100 mg/kg/day and VCR at 0.2 mg/kg twice weekly, both starting 4 days post-transplant. Mantel-Cox test p values: control versus VCR p = 0.1690, control versus GDC0941 p = 0.0645, VCR versus GDC0941 p = 0.1721, VCR versus combination p = 0.0290, GDC0941 versus combination p = 0.1762, and control versus combination p = 0.0067.
(F) Analysis of bone marrow cells harvested at the time of euthanasia from representative recipients of the indicated therapy.
