Abstract
Introduction
The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial assessed the effect of screening with prostate-specific antigen and digital rectal exam on prostate cancer (PCa) mortality. Another endpoint of interest is the burden of total metastatic disease.
Methods
All men in PLCO were assessed for metastatic PCa at diagnosis; men with clinical stage I/II disease were assessed for metastatic progression. The rate of total metastatic disease was defined as metastases found either at diagnosis or through progression divided by person years (PYs) of follow-up for all trial men. Metastatic progression rates were computed among men with clinical stage I/II PCa. Survival among men with metastases at diagnosis was compared to survival for men with metastatic progression.
Results
Among 38340 (intervention arm) and 38343 (control arm) men in PLCO, there were 4974 (intervention) and 4699 (control) PCa cases. The rate (per 10,000 PY) of total metastatic disease was 4.72 (intervention arm) versus 4.83 (control arm); RR=0.98; 95% CI: 0.81–1.18. The rate of metastatic progression (per 10,000 PY) among men with clinical stage I/II PCa was 43.7 and 50.5 in the intervention and control arms, respectively (p=0.36). Prostate-cancer specific 5- and 10-year survival was significantly worse for men with metastatic progression (24% and 19%) than for men with metastases at diagnosis (40% and 26%).
Conclusion
Rates of total metastatic disease and of metastatic progression were similar across arms in PLCO. Survival was worse for men with metastatic progression compared to those with metastatic disease at diagnosis.
Keywords: prostate cancer, screening, metastatic disease, metastatic progression
Precis:
In the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer screening trial, the total rate of metastatic prostate cancer, combined at diagnosis and through progression, was similar across trial arms. Rates of metastatic progression were similar across arms, but increased with increasing PSA and Gleason score.
Introduction
For the past three decades, screening with prostate-specific antigen (PSA) has been a defining feature of the landscape of prostate cancer. Although there have been three large-scale PSA screening trials, and decades of observational data, the jury is still out as to whether the benefits of PSA screening outweigh the harms 1. The primary goal of PSA screening is to reduce mortality from prostate cancer, and the primary endpoint of the three screening trials was prostate cancer mortality 2–5. One of these trials, the European Randomized Study of Screening for Prostate Cancer (ERSPC), found a significant reduction in prostate cancer mortality 2. In contrast, neither of the other two trials, the U.S.-based Prostate, Lung, Colorectal and Ovarian (PLCO) trial and the UK CAP trial, found a significant mortality reduction, although a high control arm contamination rate in PLCO and a low screening arm compliance rate in CAP resulted in a reduced statistical power for these trials 3–7.
An important additional goal of PSA screening is reducing the rate of metastatic prostate cancer, and this was a secondary endpoint of the screening trials. Besides being a surrogate endpoint for mortality, metastatic disease is an important clinical outcome in its own right, given the adverse impact on quality of life of the disease and its treatments, whether the metastases are present at diagnosis or occur later through progression 8–9. The ERSPC analyzed rates of total metastatic disease (metastases at diagnosis plus metastatic progression) by arm in a subset of its centers and showed a reduction similar to, but slightly larger than, the reduction in prostate cancer mortality in the trial 10. The CAP trial reported only on metastatic disease at diagnosis (M1), grouped together with N1 and T4 disease, and showed a statistically significant reduction in T4, N1 or M1 disease in the screening versus control arm 3. PLCO previously reported rates of metastatic disease at diagnosis by trial arm, with no significant differences between arms, but not on metastatic progression or total metastatic disease 5.
To assess rates of total metastatic disease in the PLCO trial, an ancillary study was conducted among men with clinically localized prostate cancer to study disease progression. In this paper, we utilize data from this ancillary study, along with standard trial data, to examine the rates of metastatic progression of clinically localized cases by trial arm and examine the rates of total metastatic disease (at diagnosis and through progression) by trial arm. In addition, we compare the survival of men with metastatic disease at diagnosis with that of men with metastatic progression.
In the screening trial context, it is especially important to compare the rate of total metastatic disease across trial arms, not just the rate of metastatic disease at diagnosis. Due to the lead time induced by screening, time of diagnosis is not independent of screening arm, and thus using an endpoint defined by time of diagnosis is subject to lead-time bias. For example, even if screening did nothing to prevent metastatic disease, a screened arm could have lower rates of metastatic disease at diagnosis than a control arm merely due to earlier diagnosis, before metastatic disease was apparent. Thus, this analysis represents an assessment of the effect of PSA screening on metastatic prostate cancer in PLCO unaffected by lead-time bias.
Methods
PLCO Design
The design of the PLCO Trial has been described 4,5. Briefly, randomization at ten U.S. screening centers of subjects aged 55–74 to either an intervention or control arm occurred from 1993–2001. The primary exclusion criteria were a history of prostate, lung, colorectal, or ovarian cancer, current cancer treatment, and beginning in 1995, having had more than one PSA blood test in the prior three years. Men randomized to the Intervention arm received a PSA test and digital rectal exam (DRE) at baseline, an annual DRE for three more years and, generally, an annual PSA for five more years. PSA results were classified as abnormal if they exceeded 4 ng/ml. DRE results were considered abnormal if there was nodularity or induration of the prostate, or if the examiner judged other criteria to be suspicious for cancer, including asymmetry. Participants and their physicians were notified in writing of any suspicious abnormality on screening. The diagnostic process following a positive screen was managed by participants’ primary care physicians, not dictated by the trial.
The original analysis period for the prostate component of the trial was from randomization through 13 years of follow-up or December 31st, 2009, whichever came first 4. For this period, incident cancers and deaths were primarily ascertained through a mailed annual study update questionnaire (ASU). Reported cancers were verified by medical records and abstracted utilizing a standardized process to determine cancer type, date of diagnosis and tumor characteristics (including stage and Gleason grade). Active follow-up was augmented by linkage to the National Death Index (NDI), and death certificates were obtained to confirm death. Subsequently, extended follow-up for mortality and cancer incidence was performed, primarily through linkages to the National Death Index (NDI) and to state cancer registries, with follow-up through the end of 2014 for cancer incidence and through the end of 2015 for mortality for the majority of subjects 5.
Prostate Cancer Progression (PCP) Ancillary Study
PCP was an ancillary study of PLCO initiated in 2010 to evaluate the progression of prostate cancer, including metastatic progression, among men diagnosed with localized disease. Men with clinical stage I or II prostate cancer diagnosed through the end of 2009 were eligible for PCP (Figure 1). For PCP-eligible men who were known to be deceased at the time of PCP, medical records were obtained and information on disease progression and treatment was collected using a standard Prostate Progression Abstracting (PPA) form. Those who were alive and agreed to participate were interviewed by trained study personnel by telephone using a standardized questionnaire that inquired about disease recurrence and progression. Specifically, the questionnaire asked whether a physician ever told them they had a recurrence, whether they had been told that the disease had spread outside of the prostate or to other parts of the body, whether their PSA had risen since diagnosis or treatment, and whether they had received additional or more aggressive treatments for prostate cancer since their initial treatment (for those with initial prostatectomy or radiation treatment) or following a period of watchful waiting or active surveillance. Medical records were obtained for those participants who responded “Yes” to any of the above four questions and information related to disease progression and treatment was abstracted using the PPA form, in the same manner as deceased men (Figure 1). With respect to metastatic progression, abstractors filled out the PPA indicating if metastases had occurred and the date of the first record of metastatic progression. They also recorded the level of nodal involvement, as well as the results of the PSA testing associated with recurrence, metastasis or additional treatment.
Figure 1.
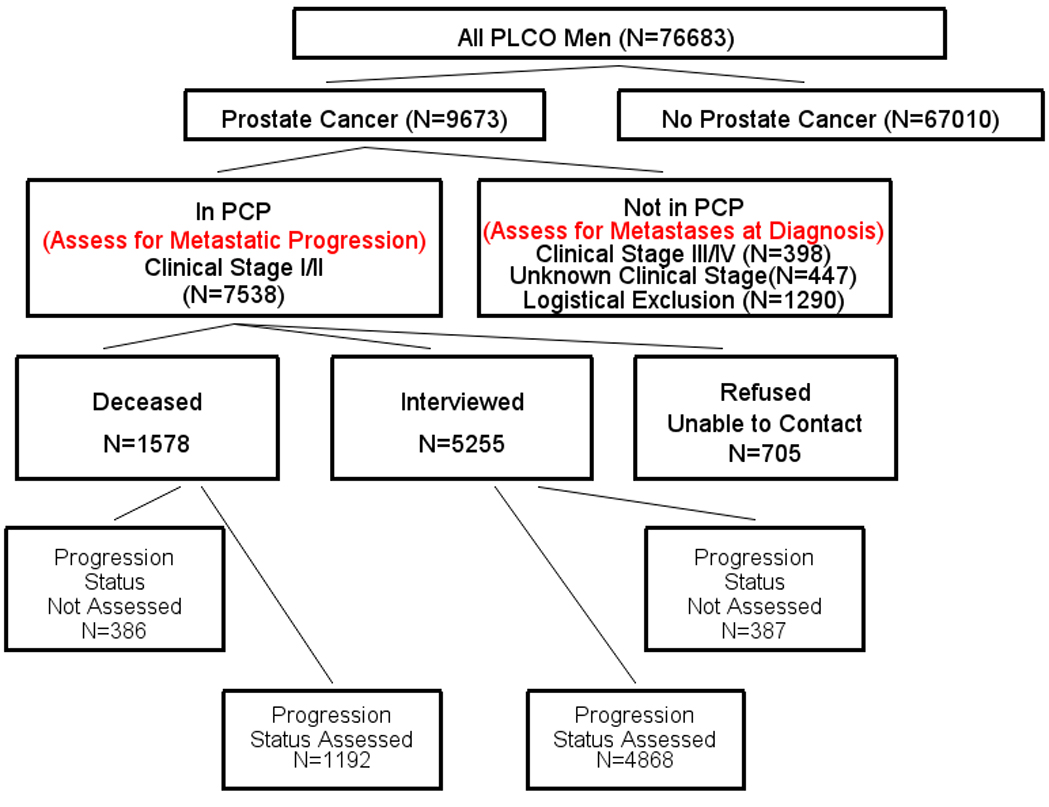
Assessment for total metastatic disease of all PLCO men (both trial arms). Total metastatic disease includes metastases at diagnosis and metastatic progression. The Prostate Cancer Progression (PCP) ancillary study examined metastatic progression in clinical stage I/II cases. Cases not included in PCP could have metastatic disease at diagnosis. Cases included In PCP were generally assessed for metastatic progression. Progression status was not assessed if medical record abstracting (PPA form) could not be performed for deceased subjects or for interviewed subjects who indicated they had a recurrence or additional treatment; status was also not assessed for those refused/unable to contact. PCP cases could have metastatic disease at diagnosis if they were pathologic stage M1 or had progression within 6 months of diagnosis (these are included as progression status not assessed). Of logistical exclusions to PCP, 53% were cases diagnosed from 2009 onward, which were generally not known to the trial at the time of selection for PCP.
Metastatic Disease
All men diagnosed with prostate cancer were assessed for metastatic disease at diagnosis. Metastatic disease at diagnosis was defined as (clinical or pathologic) stage M1 disease, with the date of metastatic disease being the diagnosis date. In addition, men without stage M1 disease but with metastatic progression (as assessed from PCP) within 6 months of diagnosis were considered to have metastatic disease at diagnosis. Information on metastatic progression was limited to those men with clinically localized disease who participated in PCP and whose metastatic progression status was assessed (Figure 1). Men were considered to have metastatic progression if they had documented evidence of metastasis (M1) on a PPA, with a date of metastatic disease the time of first record of metastatic progression. Men not reporting any signs or symptoms of disease progression on the interview or not having evidence of metastatic disease on their PPA were assumed to have no metastatic progression. Men who reported progression or died, but for whom medical records could not be obtained, were considered to have unknown progression status (Figure 1). Similarly, men not part of PCP, either for logistical reasons (e.g., not known to be a case at time of initiation of PCP) or because they were not clinical stage I/II, were considered to have unknown metastatic progression. Total metastatic disease was defined as metastatic disease at diagnosis or through progression.
Quantitative Methods
To compute the incidence rate of total metastatic disease by trial arm, all metastatic events through a cutoff date of 12/3½010 were included. With this cutoff date, 97% of metastatic events identified in PCP were included in the analysis and 90% of PCP participants had complete follow-up for progression through the cutoff. Further, follow-up for prostate cancer initial diagnoses (and metastases at diagnosis) was reasonably complete through this cutoff date; 84% complete through the end of 2010 and 98% complete through the end of 2009.
All men were included in the analysis of total metastatic disease incidence, with follow-up time beginning at randomization. Men without documented prostate cancer by the cutoff date were censored at that time or death, whichever came first. For men with metastatic disease (either at diagnosis or through progression), the end of follow-up was the date of metastatic disease. For those with a prostate cancer diagnosis but no reported metastatic disease, censoring occurred at the cutoff date or death (whichever came first) for men in PCP with known (negative) metastatic progression status and at their prostate cancer diagnosis date for men either not in PCP or in PCP but with metastatic progression not assessed. Person years at risk for total metastatic disease were computed using the above follow-up times; rates of total metastatic disease were computed, by trial arm, as number of subjects with metastatic disease (at diagnosis or through progression) per 10,000 PYs at risk. Kaplan-Meier analyses were performed to compare cumulative rates of total metastatic disease by arm 11.
Rates of metastatic progression were assessed among all men in PCP with known metastatic progression status (Figure 1). Rates were computed using the same cutoff date and end of follow-up times as above; since progression within 6 months of diagnosis was considered metastatic disease at diagnosis, follow-up time began 6 months after diagnosis. Progression rates were computed as number of men with metastatic progression per 10,000 PYs at risk. Kaplan-Meier curves were utilized to estimate cumulative progression probabilities over time. Analyses were performed overall, by trial arm and by tumor characteristics.
We also examined survival rates for all men with either metastatic disease at diagnosis or metastatic progression. For metastatic disease at diagnosis, survival was measured from time of diagnosis, while for those with metastatic progression, survival was measured from time of first record of metastases. The end date for survival was the end of extended follow-up for mortality. Kaplan-Meier curves and univariate and multivariate proportional hazards models were utilized to examine survival. For the multivariate model, covariates included age, race and biopsy Gleason score.
In addition, to assess the representativeness of the men with metastatic disease at diagnosis in PLCO, we computed expected survival rates for PLCO using survival data from the SEER database and compared them to observed rates 12. Specifically, prostate-cancer specific survival rates for men with metastatic disease (at diagnosis) by calendar year of diagnosis and age group were extracted from the SEER database and expected survival rates were computed taking a weighted average of SEER rates, with weights proportional to the distribution in PLCO of year of diagnosis and age group.
Results
A total of 76,683 men were enrolled in PLCO, 38,340 in the intervention and 38,343 in the control arm. Median age at randomization was 63 years; 85% were non-Hispanic white. During the incidence follow-up period through 2010, there were 9,673 prostate cancer cases diagnosed - 4,974 in the intervention arm and 4,699 in the control arm (Table 1).
Table 1.
Characteristics of prostate cancer cases, overall and in PCP study
| Intervention Arm | Control Arm | Both Arms | |
|---|---|---|---|
| All Cases Through 2010 | |||
| N=4974 | N=4699 | N=9673 | |
| Biopsy Gleason, N(%) | |||
| 2–6 | 2923 (62.4) | 2455 (56.8) | 5378 (59.7) |
| 7 | 1259 (26.9) | 1296 (30.0) | 2555 (28.4) |
| 8–10 | 499(10.7) | 571 (13.2) | 1070 (11.9) |
| Unknown | 293 | 377 | 670 |
| PSA at Diagnosis (ng/ml), median (IQ Range) | 5.9 (4.4–8.6) | 6.4 (4.8–9.7) | 6.1 (4.6–9.0) |
| Age at Diagnosis, median (IQ range) | 69 (65–73) | 70 (65–74) | 69 (65–74) |
| In PCP study with known metastatic progression status (Cases through 2010; all clinical stage I/II) | |||
| N=3235 | N=2825 | N=6060 | |
| Biopsy Gleason, N(%) | |||
| 2–6 | 2116 (66.5) | 1700 (61.0) | 3816 (63.9) |
| 7 | 798 (25.1) | 807 (29.0) | 1605 (26.9) |
| 8–10 | 270 (8.5) | 279 (10.0) | 549 (9.2) |
| Unknown | 51 | 39 | 90 |
| PSA at Diagnosis (ng/ml), median (IQ range) | 5.9 (4.5–8.3) | 6.4 (4.8–9.0) | 6.1 (4.6–8.6) |
| Age at Diagnosis, median (IQ range) | 68 (64–72) | 69 (65–73) | 68 (64–73) |
Note: Percentages for Gleason score exclude unknowns
Of these 9673 prostate cancer cases, 7538 were clinical stage I/II and selected for inclusion in the PCP study (Figure 1). Those not selected for PCP included 398 clinical stage III/IV cases, 447 cases with unknown clinical stage, and 1290 cases not known to the trial at the time of PCP initiation or with other logistical reasons for exclusion. Of cases included in PCP, 6060 (80%) had known metastatic progression status, 1192 deceased subjects and 4868 interviewed subjects (Figure 1); by trial arm these included 3235 in the intervention arm and 2825 in the control arm. Table 1 shows Gleason scores and median diagnostic PSA for cases by arm overall and among those included in PCP and assessable for progression. Of the latter, across both arms, 27% were Gleason 7 and 9% Gleason 8–10; median PSA at diagnosis was 6.1 ng/ml.
Incidence of Total Metastatic Disease by Arm Among All Men
Table 2 shows the number of cases of metastatic disease among all 76,683 men in the trial, either at diagnosis or through progression, by arm. Median (25th/75th) follow-up time for total metastatic disease was similar across arms, 12.9 (10.8/14.5) and 12.8 (10.8/14.5) years in the intervention and control arms, respectively. A total of 220 metastatic cases were observed in the intervention arm versus 224 in the control arm, giving a rate ratio (RR) of 0.98 (95% CI: 0.81–1.18; p=0.81). Figure 2 shows Kaplan-Meier curves for the cumulative probability of developing metastatic disease (at diagnosis or through progression). The 15-year cumulative probability for developing any metastases was 0.76% for the intervention arm versus 0.80% for the control arm (p=0.79, log-rank test).
Table 2.
Metastatic (M1) disease at diagnosis and through progression among all PLCO men, by arm.
| Intervention arm (N=38,340) | Control arm (N=38,343) | Rate Ratio (95% CI) | |
|---|---|---|---|
| Total PYs 1 | 466,079 | 463,950 | |
| N (rate per 10,000 PY) 1 | N (rate per 10,000 PY) 1 | ||
| Metastatic Disease at Diagnosis | 115 (2.47) | 126 (2.72) | 0.91 (0.70–1.17) |
| Progression to metastatic disease | 105 (2.25) | 98 (2.11) | 1.07 (0.81–1.41) |
| Total metastatic disease | 220 (4.72) | 224 (4.83) | 0.98 (0.81–1.18) |
Rate is for all men in the arm; Total PYs is total person years at risk for metastatic disease (at diagnosis or through progression).
Figure 2.
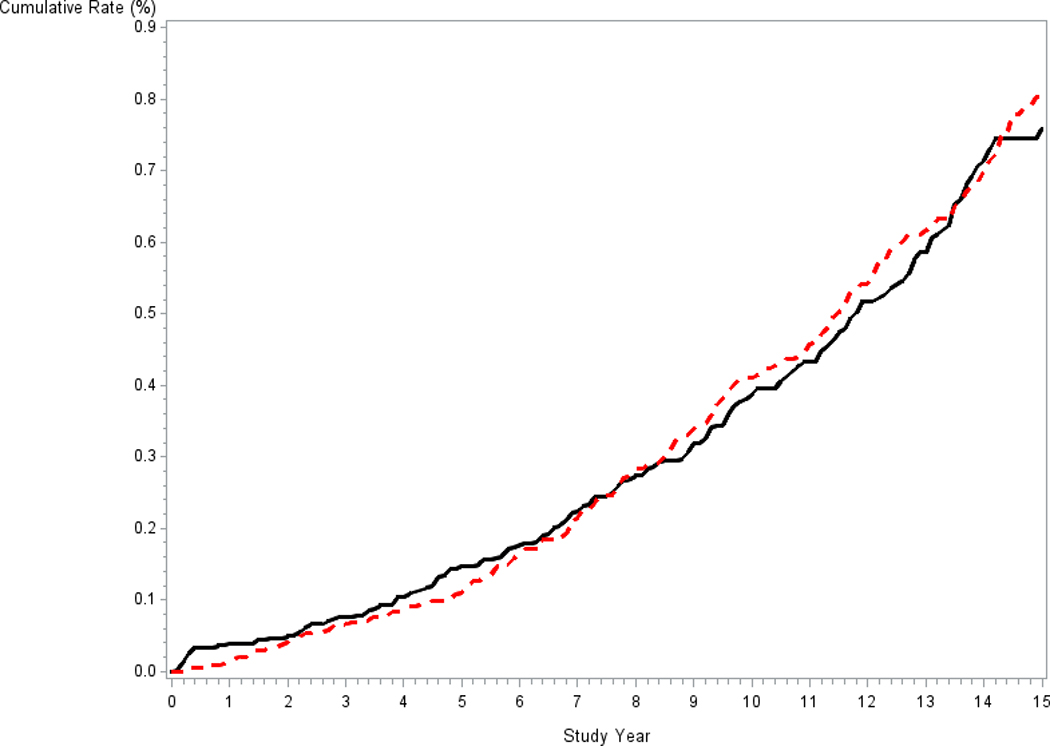
Cumulative rate of total metastatic disease (at diagnosis or by progression) among all PLCO men by arm. Solid black line is intervention arm, dotted red line is control arm.
Rates of Metastatic Progression Among Men with Clinical Stage I/II Disease
Table 3 shows metastatic progression rates among the 6060 clinical stage I/II cases with known metastatic progression status in PCP. Median (25th/75th) follow-up for progression was 7.6 (4.5/10.8) and 7.0 (4.2/9.9) years in the intervention and control arms, respectively. A total of 203 men had metastatic progression. The overall rate of metastatic progression (per 10,000 PY) was 46.8 (95% CI: 40–53). There was no significant difference in progression rate by trial arm (43.7 intervention vs. 50.5 control; RR=0.87; 95% CI: 0.65–1.14). Kaplan-Meier analysis showed a cumulative probability of progression at 10 years of 4.7% and 5.0% for the intervention and control arms, respectively, and 4.9% for both arms combined. The type of metastatic disease on progression was similar across arms; 6% M1A (non-regional lymph nodes), 78% M1B (bone) and 16% M1C (other site(s) with or without bone disease) in the intervention arm versus 2% M1A, 80% M1B and 18% M1C in the control arm. The risk of metastatic progression increased with biopsy Gleason score; progression rates (per 10,000 PY) were 20.6 (95% CI: 15–26.), 78.6 (95% CI: 61–96) and 182.7 (95% CI: 137–229) for Gleason categories 2–6, 7 and 8–10, respectively (p < 0.0001) (Table 3). Metastatic progression rates also increased with increasing diagnostic PSA level, with rates of 27.7 (95% CI: 20–35), 52.9 (95% CI: 41–65) and 79.2 (95% CI: 59–99) for PSA <6, 6–10 and > 10 ng/ml, respectively (p < 0.0001). Within each Gleason and PSA category, progression rates did not significantly differ by trial arm.
Table 3.
Rates of metastatic progression among men with clinical stage I/II disease
| Group | Number at Risk | Number with Progression | Rate per 10,000 PY (95% CI) | Rate Ratio (95% CI) | 10 Year probability of metastatic progression (95% CI), % |
|---|---|---|---|---|---|
| All | 6060 | 203 | 46.8 (40–53) | N/A | 4.9 (4.1–5.6) |
| Control Arm | 2825 | 98 | 50.5 (41–61) | Ref | 5.0 (3.9–6.1) |
| Intervention Arm | 3235 | 105 | 43.7 (35–52) | 0.87 (0.65– 1.14) | 4.7 (3.7–5.7) |
| Gleason Category 1 | |||||
| Gleason 2–6 | 3816 | 60 | 20.6 (15–26) | Ref | 2.1 (1.5–2.7) |
| Gleason 7 | 1605 | 79 | 78.6 (61–96) | 3.8 (2.7–5.4) | 8.3 (6.3–10.3) |
| Gleason 8–10 | 549 | 61 | 182.7 (137–229) | 8.8 (6.2–12.7) | 17.2 (12.7–21.7) |
| PSA at Diagnosis 2 | |||||
| <6 ng/ml | 2803 | 55 | 27.7 (20–35) | Ref | 3.0 (2.1–3.9) |
| 6–10 ng/ml | 1876 | 71 | 52.9 (41–65) | 1.9 (1.3– 2.7) | 5.3 (4.0–6.7) |
| >10 ng/ml | 1062 | 61 | 79.2 (59–99) | 2.9 (2.0– 4.2) | 8.4 (6.1–10.7) |
Based on biopsy Gleason score. 90 men had unknown Gleason score, of whom 3 had progression.
319 men had unknown diagnostic PSA, of whom 16 had progression.
The majority of men assessed for metastatic progression received treatment with curative intent, with treatments similar across arms. Radical prostatectomy was performed in 41% and 38% of intervention and control arm cases, respectively, and 40% (intervention) versus 43% (control) of cases were treated with radiation.
Of all men (N=203) with metastatic progression, 151 (74%) had received initial curative treatment (radiation or prostatectomy). Sixty-eight percent of the 203 received hormone treatment prior to metastatic progression, with 41 (20.2%) receiving delayed (> 12 months from diagnosis) hormone treatment and 98 (48.3%) receiving initial hormone treatment, with or without radiation.
Survival Following First Record of Metastasis
Figure 3 shows survival curves for metastatic cases at diagnosis (N=241) versus cases with metastatic progression (N=203), with follow-up for survival starting at diagnosis for the former and at first record of metastatic progression for the latter. Median (25th/75th) age at start of follow-up was 72 (68/78) and 74 (70/79) years for men with metastases at diagnosis and through progression, respectively. Prostate cancer-specific survival was significantly better for men with metastases at diagnosis compared to men with metastatic progression (p=0.0003, log-rank test). Five and ten-year prostate cancer-specific survival rates for men with metastases at diagnosis were 40% and 26%, respectively, compared to 24% (5 years) and 19% (10 years) for men with metastatic progression. Observed five and ten-year survival rates for men with metastatic disease at diagnosis were close to the expected survival rates, based on SEER, of 36% and 22%, respectively.
Figure 3.
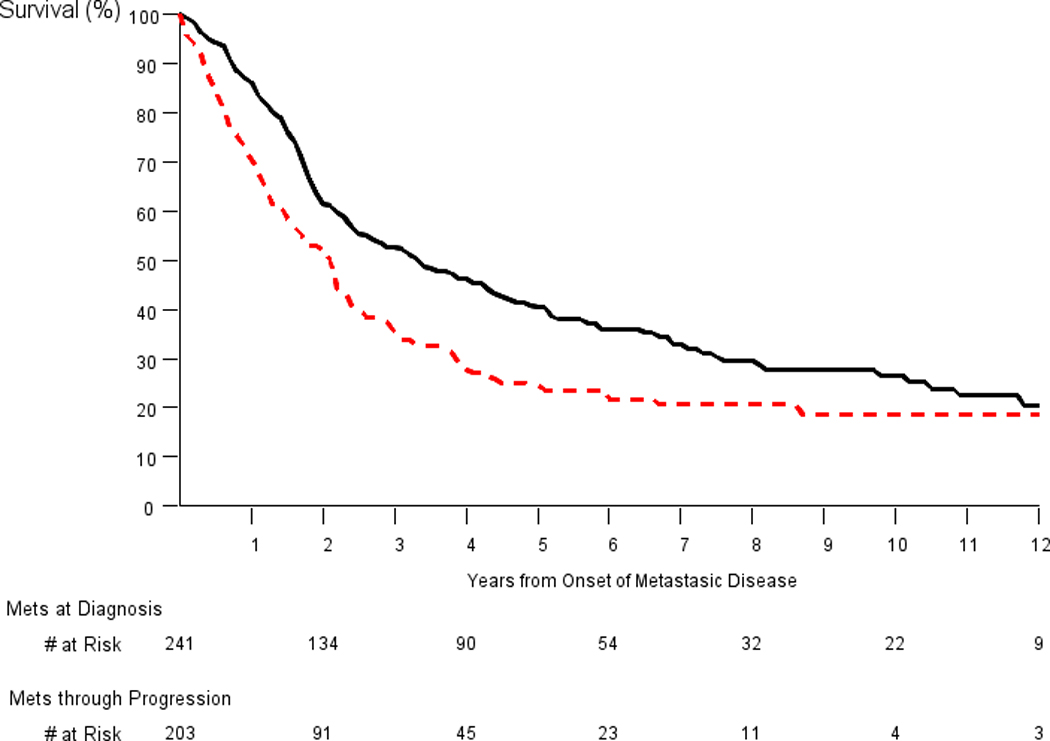
Prostate-cancer specific survival for men with metastatic disease at diagnosis (black solid line) and metastatic progression (red dotted line). Follow-up for survival began at diagnosis for the former and at first record of progression for the latter. P-value for comparison of groups equals 0.0003 (log-rank test).
All-cause survival rates were also significantly better for the group with metastases at diagnosis (p=0.0005; log-rank test); ten-year survival rates were 15% (metastases at diagnosis) versus 8% (metastatic progression). The proportional hazards model estimated a univariate HR for prostate cancer death of 1.5 (95% CI: 1.2–1.9) and a multivariate HR (controlling for age, race and Gleason score) of 1.9 (95% CI: 1.5–2.4) for men with metastatic progression versus men with metastatic disease at diagnosis.
Within the metastatic progression group, men with hormonal treatment prior to the onset of metastases had significantly worse survival compared to men with no prior hormonal treatment; 5- and 10-year prostate-specific survival rates were 17% and 10%, respectively, for men with prior hormonal treatment versus 40% and 34%, respectively, for men with no prior hormonal treatment (p = 0.0001). Proportional hazards modeling showed a univariate HR for prostate cancer death of 2.1 (95% CI: 1.4–3.0; p=0.0002) for men with prior hormonal treatment versus men with no such treatment; after controlling for the presence of initial treatment with curative intent, the HR was similar (2.0; 95% CI: 1.4–2.9; p=0.0003).
Discussion
In this analysis of the PLCO trial, the incidence of total metastatic prostate cancer (at diagnosis and through progression) was similar across arms, with an RR of 0.98 (95% CI: 0.81–1.18). Approximately half of metastatic cases were at diagnosis and half through progression. In comparison, the ERPSC trial, in the four centers where data on metastatic progression were available, found a significant reduction in metastatic disease (combined at diagnosis and through progression) in the intervention versus control arm, reporting a hazard ratio of 0.70 (95% CI: 0.60–0.82) 10. Cumulative incidence rates of total metastatic disease in ERSPC at a median of 12 years of follow-up were 0.67% (intervention) and 0.86% (control), as compared to cumulative rates through 15 years in PLCO of 0.76% (intervention) and 0.80% (control).
In a screening trial, the number of cases of metastatic disease at diagnosis would be expected to be lower in the intervention arm, even if screening didn’t prevent metastases, merely due to earlier detection, at a time point where metastases were not clinically evident. Here the number of such cases was indeed lower in the intervention arm, though not statistically significantly so (RR=0.91; 95% CI: 0.70–1.17). With respect to cases with metastatic progression by arm, two factors could be at play. First, based on the timing of diagnosis as described above, the number of men with metastatic progression could be greater in the intervention arm since a man diagnosed early through screening and still getting metastatic disease later would be a case of metastatic progression, but the same man not undergoing screening would likely be a case of metastases at diagnosis, presuming the metastatic symptoms caused the initial diagnostic work-up. Alternatively, if screening actually prevented metastatic disease through early diagnosis and treatment, this would decrease the number of intervention arm subjects with progression. Here, the number of cases with progression was similar across arms (slightly higher in the intervention arm), which could indicate that both of the above processes were operating to some extent.
In addition to similar absolute numbers, the metastatic progression rate from clinical stage I/II disease was similar across arms, with a 10-year cumulative probability of about 5%. Although progression rates increased sharply with higher Gleason scores, the absolute number of cases with progression was similar for Gleason 2–6 as for Gleason 8–10 cases, due to the much greater numbers of the former.
We found that survival from onset of metastases was significantly worse for men with metastatic progression than for men with metastatic disease at diagnosis. A possible explanation for this is that most men with metastatic progression had prior hormone therapy. Prostate tumors are known to progress under hormonal treatment of sufficient duration to an androgen insensitive state where hormonal therapy is no longer effective 13. In contrast, men with metastases at diagnosis had not received prior hormone treatment. The subset of men with metastatic progression who had not received prior hormonal treatment had similar survival rates (40% and 34% at 5- and 10-years) to men with metastases at diagnosis (40% and 26% at 5- and 10-years).
It should also be noted that men already diagnosed with (non-metastatic) prostate cancer may be more likely to undergo more careful and frequent monitoring than men without a prostate cancer diagnosis. Therefore, metastatic disease in these men may be more likely to be discovered earlier in its course than metastatic disease detected at diagnosis. If this is true, then there would be some lead-time bias operating in the metastatic progression group, implying that the observed survival difference between metastatic disease at diagnosis versus progression may be an underestimate of the true difference.
There is literature from both treatment trials and non-research settings on prostate cancer-specific survival rates following metastatic progression 14,15. Additionally, there are population-based data (from SEER) on survival rates in men with metastatic prostate cancer at diagnosis 12. However, this is the first study to our knowledge to directly compare survival among patients drawn from the same population by type of metastatic presentation, at diagnosis versus through progression. Since the two groups of men are demographically similar and were treated in similar settings at similar times, this strengthens the conclusion of a true difference in survival among men with metastatic disease at diagnosis and men with metastatic progression.
This study had several limitations. A limitation of the analysis of cumulative rates of metastatic disease across trial arms was that men with clinical stage III/IV disease but without evidence of metastases (at diagnosis) were excluded from the PCP study and hence not tracked for metastatic progression. However, the number of such men was similar across arms, 85 in the intervention versus 100 in the control arm. Therefore, even though these men would likely have higher metastatic progression rate than men with clinical stage I/II disease, who were tracked for progression, the small difference in their numbers across trial arms suggests that having included these men would have resulted in little change, and perhaps a slight decrease, in the reported RR of 0.97 for metastatic disease. A related limitation is that some men targeted for inclusion in PCP declined to participate or could not be contacted, and medical records could not be abstracted for some. In total, men missing data on progression were distributed similarly across arms, with 18.7% of intervention arm versus 19.3% of control arm cases targeted for PCP inclusion having missing progression data. Therefore, the effect of this missing data on the RR was likely minimal. Additionally, some clinical stage I/II cases were not included in PCP, primarily because they were diagnosed and identified too late for inclusion, but the non-inclusion rate was similar across arms, 13.0% and 16.4% in the intervention and control arms respectively. Finally, as has been noted previously, there was substantial contamination (use of PSA screening) in the PLCO control arm. During the screening phase, 46% of control arm men received a PSA screen in the prior year, compared to 84% of intervention arm men, and through 13 years of follow-up 88% of control versus 99% of intervention arm men had received any PSA testing 6,16. This reduced the power of the trial to find a significant prostate cancer mortality difference across arms and also likely reduced the power to find a significant difference across arms in total metastatic disease.
With respect to the analysis of survival from time of metastases, the analyzed cohort with metastatic progression excluded men with non-metastatic clinical stage III/IV disease since these men were not tracked for progression. However, since men with metastatic progression had worse survival than those with metastases at diagnosis, and since it is unlikely that men with clinical stage III/IV who progressed to metastases would have better survival than men with clinical stage I/II disease who so progressed, having included in the survival analysis those clinical stage III/IV cases who progressed would not likely change the conclusion that men with metastatic progression had worse survival than men with metastases at diagnosis.
Conclusion
The overall rate of metastatic disease, combined at diagnosis and through progression, was similar in in the PLCO intervention compared to control arm. Across both arms, compared to men with metastases at diagnosis, survival was worse for men with metastatic progression, and specifically for the subset of men with progression who received prior hormone therapy.
Acknowledgments
Funding: This work was supported by NIH contracts N01-CN-25476 and N02-CN-63300 and the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, NCI, NIH.
Footnotes
Conflicts of Interest: All authors report no conflicts of interest.
References
- 1.Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319: 1901–1913. [DOI] [PubMed] [Google Scholar]
- 2..Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384: 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin RM, Donovan JL, Turner EL, et al. Effect of low-intensity PSA-based screening intervention on prostate cancer mortality: The CAP randomized clinical trial. JAMA 2018; 319: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andriole GL, Crawford ED, Grubb RL et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial: Mortality results after 13 years of follow-up. J Natl Cancer Inst 2012; 104: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinsky PF, Miller E, Prorok P, et al. Extended follow-up for prostate cancer incidence and mortality among participants in the Prostate, Lung, Colorectal and Ovarian randomized cancer screening trial. BJU Int 2018; doi: 10.1111/bju.14580 [DOI] [PMC free article] [PubMed]
- 6.Pinsky PF, Black A, Kramer BS, et al. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Clinical Trials 2010; 7: 303–311. [DOI] [PubMed] [Google Scholar]
- 7.Pinsky PF. Power of a trial investigating a low-intensity PSA-based screening intervention. JAMA 2018; 320: 600. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins V, Solis-Trapala I, Payne H, et al. Treatment experiences, information needs, pain and quality of life in men with metastatic castrate-resistant prostate cancer: results from the EXTREQOL Study. Clin Oncol 2019; 31: 99–107. [DOI] [PubMed] [Google Scholar]
- 9.Holmstrom S, Naidoo S, Turnbull J, et al. Symptoms and impacts in metastatic castration-resistant prostate cancer: qualitative findings from patient and physician interviews. Patient 2019; 12: 57–67. [DOI] [PubMed] [Google Scholar]
- 10.Schroder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2012; 62: 745–752. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958:
- 12.Surveillance, Epidemiology and End Results (SEER). Cancer Statistics Review http://seer.cancer.gov/csr/1975_2013/. Accessed July 5th, 2016.
- 13.Eisenberger MA, Simon R, O’Dwyer PJ, et al. A reevaluation of nonhormonal cytotoxic chemotherapy in the treatment of prostatic carcinoma. J Clin Oncol 1985; 3: 827–841. [DOI] [PubMed] [Google Scholar]
- 14.Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol 2016; 34: 1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascale M, Azinwi CN, Marongiu B, et al. The outcome of prostate cancer patients treated with curative intent strongly depends on survival after metastatic progression. BMC Cancer 2017; 17: 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinsky PF, Prorok PC, Yu K, et al. Extended Mortality Results for Prostate Cancer Screening in the PLCO Trial with Median 15 Years Follow-up. Cancer 2017; 123: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]


