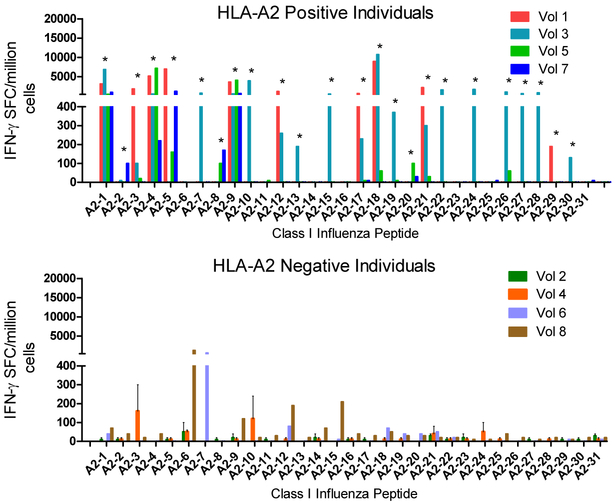
Figure 4. Human immunogenicity of highly conserved HLA-A2 restricted influenza A epitopes.
PBMC from HLA-A2 positive and HLA-A2 negative individuals were cultured with DMSO alone or with individual putative influenza A HLA-A2-restricted epitopes for 2 weeks with IL-2 provided on days 0, 2, 7 and 10. On day 14 of culture, cells were washed and added to IFN-γ ELISPOT assays with the same peptide as utilized for expansion. Shown are results [expressed as spot forming cells (SFC) per million cells] obtained from PMBC collected from 4 HLA-A2 positive (A-top) and 4 HLA-A2 negative (B-bottom) individuals (DMSO expansion subtracted). Data shown represent means ± standard errors for each volunteer. Positive responses are marked with asterisks as defined in Methods.