Abstract
The newly identified Src homology and collagen (Shc) family member ShcD was observed to be upregulated in 50% of vertical growth phase and metastatic melanomas. The aim of the present study was to investigate the mechanism by which ShcD mediates cell motility. 293 cell lines were altered to stably express GFP (GF) or GFP-ShcD (G5). Treatment of the cells with transforming growth factor (TGF)β2 promoted extracellular signal-regulated kinase (ERK) phosphorylation and, to a lesser extent, Smad2 phosphorylation in GFP-ShcD-expressing cells but not in GFP-overexpressing cells. GFP-ShcD-expressing cells exhibited upregulated expression of certain epithelial-mesenchymal transition-related genes, such as snail family transcriptional repressor 1 and SLUG, than GFP-expressing cells. Higher levels of ERK were found in the nuclear fraction of GFP-ShcD-expressing cells than that of GFP-expressing cells. Overall, GFP-ShcD-expressing cells demonstrated enhanced migration compared with GFP-expressing cells. A slight increase in cell migration was observed in both cell lines (GF and G5) when the cells were allowed to migrate towards conditioned medium derived from TGFβ2-treated GFP-ShcD expressing cells. Collectively, ShcD upregulation was proposed to induce cell migration by affecting the expression of certain epithelial-mesenchymal transition-related genes. Thus, our findings may improve understanding of the role of ShcD in cell migration.
Keywords: Src homology and collagen D, transforming growth factor β2, ERK, epithelial-mesenchymal transition, snail family transcriptional repressor 1, snail family transcriptional repressor 2
Introduction
The Src homology and collagen (Shc) family of adaptor proteins assists in propagating extracellular signals to the internal cellular environment by acting as a substrate for different receptors (1–3). In addition, Shc proteins are known for their role in establishing crosstalk between different signalling pathways (4,5). Shc proteins are capable of performing this transduction function as they possess a unique structural homologue [collagen homology (CH)2-phosphotyrosine binding domain (PTB)-CH1-Src homology-2 (SH2)] (4,5). The family comprises four members: ShcA, ShcB, ShcC and the most recently identified member ShcD (4,6,7). Expression analysis has revealed high levels of ShcD in the vertical growth phase, as well as in metastatic melanoma (6). ShcD has been proposed to mediate cell motility by activating both Ras-dependent and Ras-independent migratory pathways in melanoma (6). In the same study, when the Ras/MAPK signalling pathway was inhibited, ShcD-overexpressing cells maintained their ability to mediate cell migration (6); the mechanisms underlying ShcD-induced cell migration have require further investigation.
For cells to acquire a motile phenotype, they initially transition to a mesenchymal phenotype, and this results in the ability to invade the extracellular matrix (8). Additionally, cancer cells require new blood vessel formation to assist in metastasis (9,10). Epithelial-mesenchymal transition (EMT) is required for normal cells to migrate to aid in embryonic development and wound healing, but it is also employed by cancer cells (9). Transforming growth factor (TGF)β signalling plays a major role in epithelial cell morphology changes and alterations in gene expression patterns to acquire a motile phenotype (11–13).
Various studies have reported that TGFβ signalling is transduced via Smad and non-Smad signalling. TGFβ binds to TGFβ receptor type II, which then transphosphorylates TGFβ receptor type I. Two of the proteins recruited by TGFβ receptor type I activation are Smad2 and Smad3, and the complex they form is responsible for Smad4 translocation to the nucleus (14,15). Smad4 promotes the transcription of different genes responsible for acquiring the EMT phenotype (16). TGFβ signalling is involved in promoting the expression of stemness genes, such as snail family transcriptional repressor 2 (SLUG) and snail family transcriptional repressor 1 (SNAIL), which help initiate the EMT process (12,17). It has been reported that TGFβ triggers cell invasion and metastasis by inducing the expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinase 2 (MMP2) (18).
In the present study, we proposed that ShcD may have a role in inducing EMT and thus mediate cell migration. Investigating the expression of EMT genes in ShcD-overexpressing cells could aid in determining the function of ShcD and improve our knowledge of the mechanisms by which ShcD induces melanoma cell migration.
Materials and methods
Antibodies and reagents
The cells were lysed using 1% Triton lysis buffer supplemented with 1mM Na3VO4, 50 mM NaF, 1 mM PMSF and protease inhibitors cocktail. All the lysis buffer reagents were obtained from Sigma Aldrich (Merck KGaA). The extracted proteins were quantified by Pierce BCA protein assay from Invitrogen (23225; Thermo Fisher Scientific, Inc.). The proteins were then resolved in a 10% SDS-PAGE gel. Immunoblotting was performed by probing proteins transferred onto PVDF membranes with the following antibodies: Anti-Smad2 (ab40855; Abcam), anti-phosphorylated-Smad2 (ab184557; Abcam), anti-histone H3 (ab1791; Abcam), anti-extracellular signal-regulated kinase (ERK; cat. no. 9102; Cell Signaling Technology, Inc. (CST)], anti-phosphorylated-ERK (pERK; cat. no. 9101; CST), Anti-TGFβ receptor I antibody (ab31013; Abcam), anti-β actin (A5441; Sigma Aldrich; Merck KGaA) and anti-green fluorescent protein (GFP; sc-9996; Santa Cruz Biotechnology, Inc.). To enable detection of the primary antibody, a rabbit or a mouse secondary antibody coupled to HRP was used according to the primary antibody species (ab7628 and ab191866, respectively; Abcam). All the primary antibodies were incubated over night at 4°C, while the secondary antibodies were incubated for 1 h at RT. The membranes were developed employing ECL Western Blotting substrate kit from Promega, USA (W1015). The images were acquired by Bio-Rad ChemiDoc touch imaging system. Human TGFβ (T2815; Sigma-Aldrich; Merck KGaA) was used for cell treatment at 5 ng/ml at 37°C.
Cell culture
293 and G5 cells were a kind gift from Dr Prigent (University of Leicester, Leicester, UK). The G5 cell line was generated by Samrein Ahmed, 2013, (University of Leicester). The GF cell line was generated by Samrein Ahmed at the University of Sharjah (UAE). The method of how stable cell lines were generated is described by Ahmed et al (19). FM-55p (13012417) and MM138 (10092321) melanoma cell lines were supplied from Sigma Aldrich (Merck KGaA) from the ECACC collection. The two cell lines were maintained as indicated by ECACC instructions.
The 293, G5, and GF cell lines were cultured in Dulbecco's Modified Eagle's medium (D6429; Sigma Aldrich; Merck KGaA) supplemented with 10% FBS (F9665; Sigma Aldrich; Merck KGaA) and 1% penicillin/streptomycin. G5 and GF cells were cultured with 200 µg/ml neomycin or hygromycin, respectively for selection. The cells were incubated at 37°C and 5% CO2. Before and during the experiments, the cells were maintained without selection pressure to eliminate any effect of the selection treatment.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using a total RNA Purification Kit 1700 (Norgen Biotek Corp.). mRNA was then converted into cDNA using a TruScript Reverse Transcriptase kit following the manufacturer's protocol (cat. no. 54440; Norgen Biotek Corp.). qPCR was performed using a SYBR Green PCR kit (204145; Qiagen GmbH) and the following primers:
Homo sapiens VEGF forward, 5′-CTACCTCCACCATGCCAAGT-3′, and reverse, 5′-GCAGTAGCTGCGCTGATAGA-3′; homo sapiens MMP-2 forward, 5′-TCTCCTGACATTGACCTTGGC-3′, and reverse, 5′-CAAGGTGCTGGCTGAGTAGATC-3′; Homo sapiens SNAIL forward, 5′-ACCACTATGCCGCGCTCTT-3′, and reverse, 5′-GGTCGTAGGGCTGCTGGAA-3′; homo sapiens SLUG forward, 5′-TGTTGCAGTGAGGGCAAGAA-3′, and reverse, 5′-GACCCTGGTTGCTTCAAGGA-3′; and homo GAPDH forward, 5′-AGGGCTGCTTTTAACTCTGGT-3′, and reverse, 5′-CCCCACTTGATTTTGGAGGGA-3′. The RT-qPCR parameters for each of the genes are demonstrated in Table I. Fluorescence signals were detected using a Qiagen Rotor Gene Q PCR fluorescence analyser (Qiagne GmbH). The obtained quantification cycle (Cq) values were analysed using the 2−ΔΔCq method (20).
Table I.
RT-qPCR parameters for the tested genes.
| Gene Name | RT-qPCR parameters |
|---|---|
| SLUG | -Denaturation at 95C for 15 min |
| −45 Cycles of: | |
| −94C for 15 sec | |
| −64C for 30 sec | |
| −72C for 30 sec | |
| -Dissociation at 60-95C | |
| SNAIL | -Denaturation at 95C for 15 min |
| −45 Cycles of: | |
| −94C for 15 sec | |
| −65.7C for 30 sec | |
| −72C for 30 sec | |
| -Dissociation at 60-95C | |
| VEGF | -Denaturation at 95C for 15 min |
| −45 Cycles of: | |
| −94C for 15 sec | |
| −60C for 30 sec | |
| −72C for 30 sec | |
| −Dissociation at 60-95C | |
| MMP2 | -Denaturation at 95C for 15 min |
| −45 Cycles of: | |
| −94C for 15 sec | |
| −61C for 30 sec | |
| −72C for 60 sec | |
| -Dissociation at 60-95C | |
| GAPDH | -Denaturation at 95C for 15 min |
| −45 Cycles of: | |
| −94C for 15 sec | |
| −58C for 30 sec | |
| −72C for 30 sec | |
| -Dissociation at 60-95C |
RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Transwell assay
Briefly, 1.25×105 cells were resuspended in DMEM containing 0.1% serum and then added to upper Boyden chambers. Conditioned medium was made by adding fresh DMEM with 10% FBS to TGFβ-treated or untreated GF, or G5 cell-derived medium at a ratio of 1:1. The lower chambers contained the conditioned medium, and the cells were allowed to migrate for 16 h at 37°C. After the incubation time, the Boyden chamber membranes were stained with 0.2% crystal violet in 10% ethanol for 30 min at room temperature, and absorbance readings were obtained at 570 nm using Thermo Scientific Varioskan Flash-Elisa microplate reader (Thermo Fisher Scientific, Inc.).
Subcellular fractionation
The steps conducted to separate the nuclear fraction from the cytoplasmic fraction are described by Ahmed and Prigent (21). Briefly, cells were pelleted at 4°C at 122 × g for 5 min and the pellets were treated with hypotonic buffer (10 mM HEPES pH 7.8, 25 mM β-glycerophosphate, 25 mM MgCl2, 0.1 mM Na3VO4, 0.5 mM EDTA and 0.1% protease inhibitors). Next, 10% NP-40 was added and accompanied with vigorous vortexing for 15 sec at room temperature. This was followed by 30 sec centrifugation at 13,000 × g at 4°C. Nuclear protein extraction was performed by adding a high salt buffer (50 mM HEPES pH 7.8, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 10% glycerol, 0.2 mM NaF, 0.2 mM Na3VO4, and 0.1% protease inhibitor cocktail). Nuclear fractions were then analyzed by western blotting.
Statistical analysis
Each experiment was performed at least twice. Western blotting band analysis was performed using ImageJ-Version 1.50b (National Institutes of Health) (22) and Excel version 365. In the present study, the error bars represent the standard error of the mean. One-tailed student tests were used to calculate statistical differences. For the analysis of SLUG expression, one-way ANOVA was used as well as Dunnett's multiple comparison test for differences between multiple groups, using GraphPad Prism 7.04 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
TGFβ receptor I expression in the generated stable cell lines
To determine the effects of ShcD on downstream TGFβ signalling, stable cell lines expressing GFP-ShcD or GFP were generated (G5 and GF, respectively), and the expression of GFP-ShcD or GFP was confirmed by immunoblotting with an anti-GFP antibody (Fig. 1). Next-generation sequencing showed that the ShcD expression level was 296 times higher in G5 cells than in GF cells (data not shown).
Figure 1.

TGFβRI expression in the generated stable cell lines. (A) 293 stably expressing GFP (GF) or GFP-ShcD (G5) were generated. Immunoblotting with an anti-GFP antibody confirmed the expression of GFP and GFP-ShcD. (B) Lysates were obtained from different melanoma cell lines (MM138 and FM-55p), and 293, GF, and G5 cells. The lysates were resolved in an SDS-PAGE gel and the immunoblotting was performed by the indicated antibodies. B-actin was used as loading control. GFP, green fluorescent protein; Shc, Src homology and collagen; TGFβRI, transforming growth factor β receptor I.
The 293, GF, G5, and two melanoma cell lines (FM-55p and MM138) were analysed to determine their TGFβ receptor type I expression profile to ensure cell responsiveness to TGFβ treatment (Fig. 1).
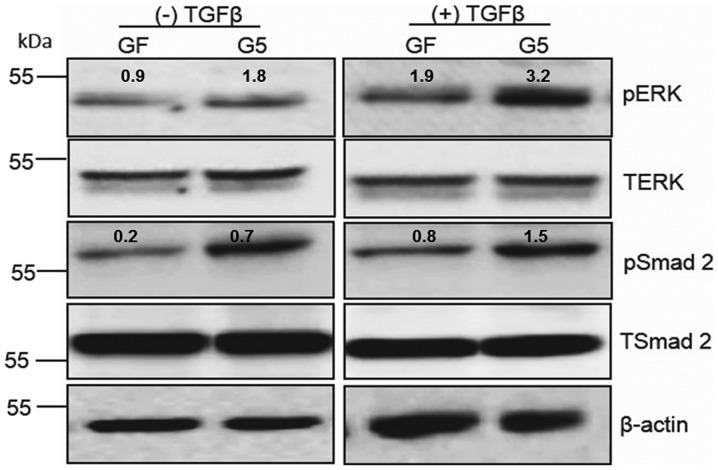
TGFβ treatment promotes ERK phosphorylation in GFP-ShcD-expressing cells
To examine whether ShcD contributes to TGFβ signalling transduction, 293, GF and G5 cells were treated with or without TGFβ. pERK was shown to be notably upregulated in GFP-ShcD-expressing cells than in GFP-expressing cells. Similar to pERK, but to a lesser extent (23), the levels of pSmad2 were increased in GFP-ShcD-expressing cells (Fig. 2). TGFβ treatment caused elevation of pERK by 1.8 fold in GFP-ShcD-expressing cells, while it resulted in increased pERK by 1-fold in GFP-expressing cells. The TGFβ-mediated Smad2 phosphorylation was less evident in GFP-ShcD-expressing cells than that of ERK phosphorylation. It was accordingly hypothesized that TGFβ treatment causes an increase in pERK and, to a lesser extent, in Smad2 phosphorylation in GFP-ShcD-expressing cells.
Figure 2.
TGFβ treatment promotes ERK phosphorylation in GFP-ShcD-expressing cells. GF and G5 cells were incubated with medium containing 0.1% FBS for 4 h. The cells were either left untreated or were treated with 5 ng/ml TGFβ2 for 1 h. The cells were then lysed and subjected to immunoblotting with the indicated antibodies. Band density analysis was performed using ImageJ software. ERK, extracellular signal-regulated kinase; p, phosphorylated; T, total; TGFβ, transforming growth factor β; GFP, green fluorescent protein..
Expression of cell motility-related genes in GF and G5 cells
The role of TGFβ in establishing and maintaining EMT was reported to be partially achieved by inducing the transcription of various genes, such as the stemness genes SLUG and SNAIL (17). Therefore, SNAIL and SLUG expression was assessed via RT-qPCR. Upon TGFβ treatment, GFP-ShcD-expressing cells exhibited significantly upregulated expression of the stemness genes with or without the addition of 10% serum (Fig. 3A and B).
Figure 3.
Expression of cell motility-related genes in GF and G5 cells. (A-D) GF and G5 cells were incubated with 0.1% serum, 0.1% serum plus 5 ng/ml TGFβ2, or complete medium plus TGFβ2 for 24 h. Total RNA was extracted, and the mRNA was then converted to cDNA. Reverse transcription-quantitative-PCR was then performed for VEGF, MMP-2, SLUG and SNAIL using Qiagen Rotor Gene Q PCR. Ns, no significance, **P<0.05. (E) Parallel sets of GF and G5 cells were treated similarly for 1 h as aforementioned. The cell lysates obtained were resolved on an SDS-PAGE gel, and immunoblotting was performed using an anti-pERK, anti-TERK or anti-β-actin antibody. TERK, total extracellular signal-regulated kinase; p, phosphorylated; SLUG, snail family transcriptional repressor 2; SNAIL, snail family transcriptional repressor 1; TGFβ, transforming growth factor β.
After cancer cells detach from neighbouring cells, resistance from the extracellular cellular matrix hinders cell motility; the secretion of extracellular proteinases, such as MMPs (23), is thus crucial for overcoming this resistance. MMP2 was found to be secreted and upregulated by various types of cancer cells to facilitate invasion (24). Furthermore, MMP2 expression was shown to be significantly increased in GFP-ShcD-expressing cells compared with corresponding GF cells when incubated with TGFβ and 10% serum but not with TGFβ alone (Fig. 3C).
Cancer metastasis is facilitated by new blood vessel formation (25). A previous report revealed that TGFβ induces angiogenesis via VEGF upregulation (26). Our findings failed to demonstrate any significant increase in VEGF gene expression in TGFβ-treated G5 cells, with or without complete medium addition, but interestingly, VEGF expression was upregulated in serum-deprived GFP-ShcD-expressing cells than in corresponding GFP-expressing cells (Fig. 3D).
In summary, SNAIL and SLUG expression was upregulated in GFP-ShcD-expressing cells in response to TGFβ treatment, unlike VEGF expression, which demonstrated higher levels when the cells were deprived of serum. MMP2 upregulation in GFP-ShcD-expressing cells was induced by the combination of 10% serum and TGFβ, but not by TGFβ alone (Table II).
Table II.
Alterations in gene expression under different conditions.
| Cell culture conditions | |||
|---|---|---|---|
| Gene | (−) TGFβ | (+) TGFβ | TGFβ + serum |
| SNAIL | −a | +b | +/−c |
| SLUG | − | + | + |
| MMP2 | − | − | + |
| VEGF | + | − | − |
Downregulation
Upregulation
Upregulation without statistical significance.
A parallel set of cells was obtained for western blotting to assess ERK phosphorylation. pERK levels were notably upregulated in GFP-ShcD-expressing cells than in control cells upon TGFβ treatment regardless to complete addition (Fig. 3E).
GFP-ShcD-expressing cells have higher nuclear levels of pERK than GF cells
GFP-ShcD-expressing cells were found to have higher expression levels of SNAIL, SLUG and MMP2 than control cells; thus, we investigated the nuclear levels of pERK upon TGFβ treatment. GF and G5 cells were either starved or treated with TGFβ with or without 10% serum. The nuclear fraction of GFP-ShcD-expressing cells exhibited higher nuclear pERK levels than that of GFP-expressing cells in starvation, TGFβ, or TGFβ treatment with complete medium conditions (P>0.05; 0.2, 0.18 and 0.09, respectively; Fig. 4A and B).
Figure 4.
GFP-ShcD-expressing cells have higher nuclear pERK levels. (A) GF and G5 cells were starved with 0.1% serum for 16 h. The cells were incubated under conditions of starvation or treated with 5 ng/ml TGFβ2 with or without 10% serum for 1 h. Subcellular fractionation was then performed to separate the nuclear fraction. Nuclear proteins were resolved on via SDS-PAGE. Immunoblotting with an anti-total ERK, anti-pERK or anti-histone antibody was performed. (B) Bar chart showing pooled data from 2 independent experiments. ERK, extracellular signal-regulated kinase; p, phosphorylated; TGFβ, transforming growth factor β; GFP, green fluorescent protein.
GFP-ShcD-expressing cells exhibit greater migration than GFP-expressing cells
To determine the migration ability of ShcD-overexpressing cells, Transwell assays were employed. The cells were allowed to migrate against a gradient created by adding conditioned medium derived from TGFβ-treated or untreated cells expressing GFP-ShcD or GFP. GFP-ShcD-expressing cells exhibited increased migration than their control counterparts, while a slight increase in cell migration towards conditioned medium derived from TGFβ-treated G5 cells was observed (Fig. 5). Despite GFP-ShcD-expressing cells showed higher migratory abilities, the data was not statistically significant.
Figure 5.
G5 cells exhibit enhanced migration than GF cells. Cells (2.5×105) were added to the upper wells of a Boyden chamber. CM with or without 5 ng/ml TGFβ2 obtained from GF or G5 cells was added to the lower chambers. The Transwell assay cultures were incubated for 16 h followed by staining with crystal violet. Absorbance readings were collected at 570 nm. CM, conditioned medium; TGFβ, transforming growth factor β.
Discussion
The role of ShcD in inducing melanoma cell migration was determined to be partially related to MAPK pathway activation (6). At present, few studies have investigated the role of ShcD in melanoma cell migration. Therefore, we aimed to determine the mechanisms underlying ShcD-mediated cell invasion and metastasis.
Stable protein expression is advantageous as the entire cell population expresses the exogenous protein, which makes it a more reliable protein expression system than others. Additionally, by analyzing stable protein expression, we generated a cellular system of ShcD upregulation, which is the mechanism by which 50% of melanomas acquire their invasive phenotype (6).
As TGFβ is a key factor in the acquisition of cell mobility, it was proposed that ShcD may exert its role in migration downstream of TGFβ signalling. This assumption regarding the Shc family is not novel as ShcA has been reported to play an essential role in non-Smad TGFβ signalling by inducing ERK phosphorylation (27–29). ERK regulates gene transcription through its downstream transcription network to mediate EMT (30–32). In the present study, SNAIL and SLUG expression levels were higher in TGFβ-treated GFP-ShcD-expressing cells than in their control counterparts. Furthermore, our findings revealed that upon TGFβ stimulation, ShcD overexpression enhanced ERK phosphorylation, subsequently inducing SLUG and SNAIL upregulation.
SLUG and SNAIL belong to the zinc finger-like family of transcription factors that are responsible for transcribing genes associated with cell migration (33,34). Previous studies have shown that the overexpression of SLUG and SNAIL is related to the invasive and metastatic phenotypes of various types of cancer, which are associated with the expression of different genes, particularly MMPs (17,35–37).
MMPs are extracellular proteases that contribute to tissue remodelling and angiogenesis, and assist in cancer cell migration (38). MMP2 upregulation is one of the molecular changes required to acquire invasion capacity in cells (39). Notably, ERK activation was reported to mediate MMP2 promoter stimulation (40). However, ERK phosphorylation was determined to lead to MMP2 upregulation, and SLUG and SNAIL were also shown to promote MMP2 upregulation (41,42). These findings could support our observation, in which we demonstrated that GFP-ShcD-expressing cells exhibited upregulated MMP2 expression upon TGFβ stimulation. In contrast to the role of TGFβ in stimulating VEGF expression (12,26), our data indicated that VEGF expression was significantly increased (P=0.04) without TGFβ treatment. This finding is supported by a previous study, which demonstrated VEGF upregulation under conditions of serum starvation in HT29 colon cancer cells via the ERK pathway (43). Notably, in this study, ERK phosphorylation was detected in the nuclei of starved G5 cells, which could explain the significant increase in VEGF expression in the serum-starved ShcD-expressing cells. These cells also demonstrated enhanced migration ability in the presence or absence of TGFβ treatment. A slight, yet not statistically significant, increase was observed with TGFβ treatment. This observation is likely due to the higher levels of phosphorylated ERK in ShcD-expressing cells regardless of TGFβ treatment. Conclusively, ShcD promoted ERK phosphorylation, which positively affected cell migration-related genes, such as SLUG, SNAIL, MMP2 and VEGF; however, only the first two genes were significantly affected by TGFβ treatment. Thus, it was proposed that ShcD acts downstream TGFβ, leading to the phosphorylation of ERK, and the subsequent induction of SNAIL and SLUG expression, promoting EMT (Fig. 6).
Figure 6.

Schematic diagram of the TGFβ-ShcD-ERK axis underlying cell migration. EMT, epithelial-mesenchymal transition; ERK, extracellular signal-regulated kinase; Shc, Src homology and collagen; SLUG, snail family transcriptional repressor 2; SNAIL, snail family transcriptional repressor 1; TGFβ, transforming growth factor β.
In the present study, ShcD was found to be associated with the migration of melanoma cells, which was achieved via MAPK activation; nevertheless, a MAPK-independent mechanism was proposed. We demonstrated that ShcD overexpression induces the expression of certain EMT-related genes by favouring crosstalk between ERK and TGFβ signalling. Furthermore, ShcD overexpression was determined to promote MMP2 upregulation. These findings suggest a novel mechanism underlying the potential of ShcD to promote cell migration and invasion.
Acknowledgements
The authors would like to thank Dr Sally Prigent (University of Leicester, Leicester, UK) for providing the 293 and G5 cell lines.
Glossary
Abbreviations
- TGFβ
transforming growth factor β
- Shc
Src homology and collagen
- MMP
matrix metalloproteinase
- VEGF
vascular endothelial growth factor
- ERK
extracellular signal-regulated kinase
- GFP
green fluorescent protein
Funding
The present study was supported by the 5th round of Bohroengir Ingelhiem grants and by a targeted grant from the University of Sharjah (grant no. 16010901011-P).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SBMA designed the experiments, performed some of the experiments, analysed the data and wrote the manuscript. SA, FAS, ZM, SM, NS and KR performed some of the experiments. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Takahashi Y, Tobe K, Kadowaki H, Katsumata D, Fukushima Y, Yazaki Y, Akanuma Y, Kadowaki T. Roles of insulin receptor substrate-1 and Shc on insulin-like growth factor I receptor signaling in early passages of cultured human fibroblasts. Endocrinology. 1997;138:741–150. doi: 10.1210/endo.138.2.4910. [DOI] [PubMed] [Google Scholar]
- 2.Polk DB. Shc is a substrate of the rat intestinal epidermal growth factor receptor tyrosine kinase. Gastroenterology. 1995;109:1845–1851. doi: 10.1016/0016-5085(95)90751-3. [DOI] [PubMed] [Google Scholar]
- 3.Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed SBM, Prigent SA. Insights into the Shc family of adaptor proteins. J Mol Signal. 2017;12:2. doi: 10.5334/1750-2187-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 6.Fagiani E, Giardina G, Luzi L, Cesaroni M, Quarto M, Capra M, Germano G, Bono M, Capillo M, Pelicci P, Lanfrancone L. RaLP, a new member of the Src homology and collagen family, regulates cell migration and tumor growth of metastatic melanomas. Cancer Res. 2007;67:3064–3073. doi: 10.1158/0008-5472.CAN-06-2301. [DOI] [PubMed] [Google Scholar]
- 7.Jones N, Hardy WR, Friese MB, Jorgensen C, Smith MJ, Woody NM, Burden SJ, Pawson T. Analysis of a Shc family adaptor protein, ShcD/Shc4, that associates with muscle-specific kinase. Mol Cell Biol. 2007;27:4759–4773. doi: 10.1128/MCB.00184-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells A, Chao YL, Grahovac J, Wu Q, Lauffenburger DA. Epithelial and mesenchymal phenotypic switchings modulate cell motility in metastasis. Front Biosci (Landmark Ed) 2011;16:815–837. doi: 10.2741/3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Son H, Moon A. Epithelial-mesenchymal transition and cell invasion. Toxicol Res. 2010;26:245–252. doi: 10.5487/TR.2010.26.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol. 2009;219:449–458. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata A, Chen YG. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8(pii):a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denis JF, Sader F, Gatien S, Villiard é, Philip A, Roy S. Activation of Smad2 but not Smad3 is required to mediate TGF-β signaling during axolotl limb regeneration. Development. 2016;143:3481–3490. doi: 10.1242/dev.131466. [DOI] [PubMed] [Google Scholar]
- 17.Naber HP, Drabsch Y, Snaar-Jagalska BE, ten Dijke P, van Laar T. Snail and Slug, key regulators of TGF-β-induced EMT, are sufficient for the induction of single-cell invasion. Biochem Biophys Res Commun. 2013;435:58–63. doi: 10.1016/j.bbrc.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 18.Kim ES, Sohn YW, Moon A. TGF-beta-induced transcriptional activation of MMP-2 is mediated by activating transcription factor (ATF)2 in human breast epithelial cells. Cancer Lett. 2007;252:147–156. doi: 10.1016/j.canlet.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed SBM, Amer S, Emad M, Rahmani M, Prigent SA. Studying the ShcD and ERK interaction under acute oxidative stress conditions in melanoma cells. Int J Biochem Cell Biol. 2019;112:123–133. doi: 10.1016/j.biocel.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed SB, Prigent SA. A nuclear export signal and oxidative stress regulate ShcD subcellular localisation: A potential role for ShcD in the nucleus. Cell Signal. 2014;26:32–40. doi: 10.1016/j.cellsig.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedl P, Bröcker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong W, Li H, Zhang Y, Yang H, Guo M, Li L, Liu T. Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim Biophys Sin (Shanghai) 2011;43:840–848. doi: 10.1093/abbs/gmr085. [DOI] [PubMed] [Google Scholar]
- 25.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 26.Fantozzi A, Gruber DC, Pisarsky L, Heck C, Kunita A, Yilmaz M, Meyer-Schaller N, Cornille K, Hopfer U, Bentires-Alj M, Christofori G. VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res. 2014;74:1566–1575. doi: 10.1158/0008-5472.CAN-13-1641. [DOI] [PubMed] [Google Scholar]
- 27.Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Northey JJ, Chmielecki J, Ngan E, Russo C, Annis MG, Muller WJ, Siegel PM. Signaling through ShcA is required for transforming growth factor beta- and Neu/ErbB-2-induced breast cancer cell motility and invasion. Mol Cell Biol. 2008;28:3162–3176. doi: 10.1128/MCB.01734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudson J, Ha JR, Sabourin V, Ahn R, La Selva R, Livingstone J, Podmore L, Knight J, Forrest L, Beauchemin N, et al. p66ShcA promotes breast cancer plasticity by inducing an epithelial-to-mesenchymal transition. Mol Cell Biol. 2014;34:3689–3701. doi: 10.1128/MCB.00341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navandar M, Garding A, Sahu SK, Pataskar A, Schick S, Tiwari VK. ERK signalling modulates epigenome to drive epithelial to mesenchymal transition. Oncotarget. 2017;8:29269–29281. doi: 10.18632/oncotarget.16493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu LY, Hsin IL, Yang TY, Sung WW, Chi JY, Chang JT, Ko JL, Sheu GT. The ERK-ZEB1 pathway mediates epithelial-mesenchymal transition in pemetrexed resistant lung cancer cells with suppression by vinca alkaloids. Oncogene. 2017;36:242–253. doi: 10.1038/onc.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng H, Li W, Wang Y, Liu Z, Cai Y, Xie T, Shi M, Wang Z, Jiang B. Glycogen synthase kinase-3 beta regulates Snail and β-catenin expression during Fas-induced epithelial-mesenchymal transition in gastrointestinal cancer. Eur J Cancer. 2013;49:2734–2746. doi: 10.1016/j.ejca.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 34.Ganesan R, Mallets E, Gomez-Cambronero J. The transcription factors Slug (SNAI2) and Snail (SNAI1) regulate phospholipase D (PLD) promoter in opposite ways towards cancer cell invasion. Mol Oncol. 2016;10:663–676. doi: 10.1016/j.molonc.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.e08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uygur B, Wu WS. SLUG promotes prostate cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol Cancer. 2011;10:139. doi: 10.1186/1476-4598-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Song GD, Sun N, Chen JQ, Yang SS. Slug overexpression induces stemness and promotes hepatocellular carcinoma cell invasion and metastasis. Oncol Lett. 2014;7:1936–1940. doi: 10.3892/ol.2014.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, Bar-Eli M, Meloche S, Brodt P. Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem. 2004;279:19683–19690. doi: 10.1074/jbc.M313145200. [DOI] [PubMed] [Google Scholar]
- 41.Merikallio H, T TT, Pääkkö P, Mäkitaro R, Kaarteenaho R, Lehtonen S, Salo S, Salo T, Harju T, Soini Y. Slug is associated with poor survival in squamous cell carcinoma of the lung. Int J Clin Exp Pathol. 2014;7:5846–5854. [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Klausen C, Zhu H, Leung PC. Activin a increases human trophoblast invasion by inducing SNAIL-mediated MMP2 Up-regulation through ALK4. J Clin Endocrinol Metab. 2015;100:E1415–E1427. doi: 10.1210/jc.2015-2134. [DOI] [PubMed] [Google Scholar]
- 43.Jung YD, Nakano K, Liu W, Gallick GE, Ellis LM. Extracellular signal-regulated kinase activation is required for up-regulation of vascular endothelial growth factor by serum starvation in human colon carcinoma cells. Cancer Res. 1999;59:4804–4807. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.