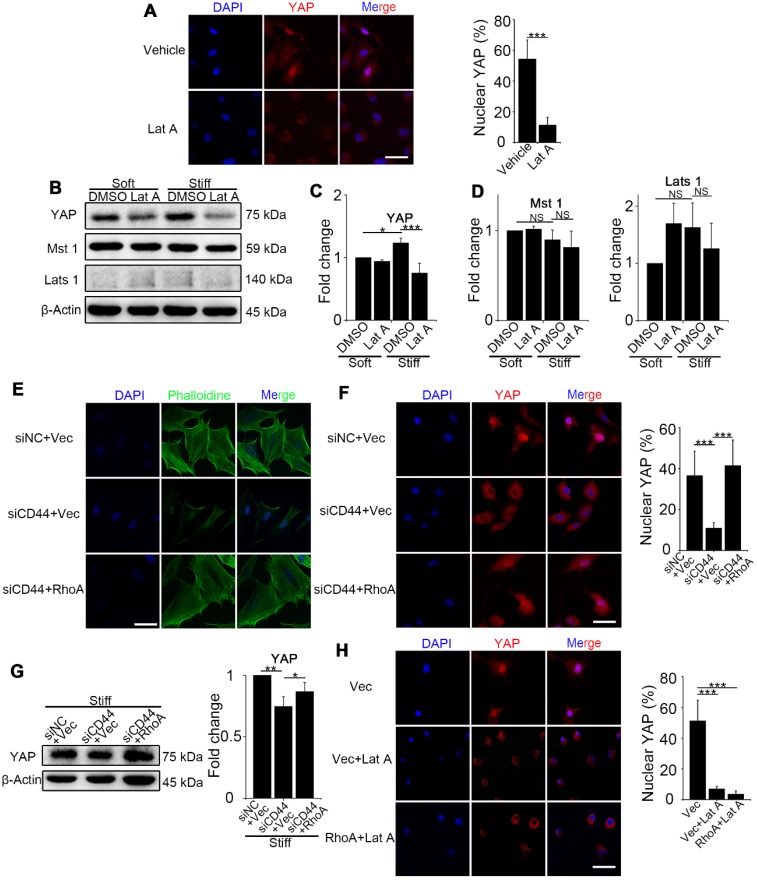
Figure 3.
RhoA acts through F-actin cytoskeleton to regulate YAP activation independent of the Hippo kinase cascade. (A-C) NIH-3T3 fibroblasts grown on stiff (60 kappa) gel-coated coverslips were treated with or without 0.5 μM Lat A for 4 hours. (A) NIH-3T3 cells were immunostained with an antibody recognizing YAP. The percentage of cells with predominantly nuclear YAP staining was quantified. Nuclei were counter-stained with DAPI (n=3; ***, P < 0.001). Scale bar, 50 μm. (B) Western blot analysis of YAP and the Hippo kinase cascade proteins (Mst 1 and Lats 1) expression. β-Actin was used as a loading control. (C-D) Quantification of the indicated level is shown. (E-G) NIH-3T3 fibroblasts grown on stiff (60 kappa) gel-coated coverslips were co-transfected with NC/CD44 siRNA and vector/constitutively active RhoA-Q63L (ca-RhoA) plasmid for 48 hours. (E, F) NIH-3T3 cells were immunostained with phalloidine to visualize F-actin (E) and an antibody recognizing YAP to visualize YAP localization (F). (n=3; ***, P < 0.001). The percentage of cells with predominantly nuclear YAP staining was quantified. Scale bar, 50 μm. (G) Western blot analysis of YAP expression. β-Actin was used as a loading control. Quantification of YAP level is shown. (H) NIH-3T3 fibroblasts grown on stiff (60 kappa) gel-coated coverslips were transfected with vector/ca-RhoA plasmid for 48 hours and treated with or without Lat A for 4 hours, and immunostained with an antibody recognizing YAP. The percentage of cells with predominantly nuclear YAP staining was quantified. Nuclei were counter-stained with DAPI (n=3; ***, P < 0.001). Scale bar, 50 μm. (C, D, G) Data shown are representative of three independent experiments. Error bars indicate mean ± SD (*, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant).