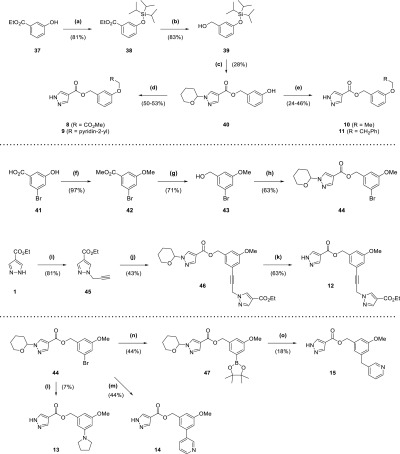
Scheme 2. Synthesis of Compounds 8–15.
Reagents and conditions: (a) TIPSCl, imidazole, DMF, 2 h; (b) LiAlH4 (2.4 M in THF), Et2O, 0 °C to rt, 3 h; (c) (i) 36, DIPEA, DMAP, EDC·HCl, DCM, overnight (ii) TBAF (1 M in THF), 10 min; (d) (i) Cs2CO3, RCH2Br (·HBr), DMF, 2 h (ii) TFA, 15 min; (e) (i) PPh3, RCH2OH, DIAD, THF, 1 h to overnight (ii) TFA, 15 min; (f) MeI, K2CO3, DMF, 3 d; (g) LiAlH4 (2.4 M in THF), Et2O, 0 °C to rt, overnight; (h) 36, DIPEA, DMAP, EDC·HCl, DCM, overnight; (i) propargyl bromide (80 wt % solution in toluene), K2CO3, DMF, 3 d; (j) 44, Pd(dppf)Cl2, CuI, NEt3, DMF, 100 °C, 4 h; (k) TFA, 15 min; (l) (i) pyrrolidine, Cs2CO3, RuPhos, RuPhos Pd G1 methyl tert-butyl ether adduct, tBuOH, 85 °C, 15 h (ii) TFA, 30 min; (m) (i) 3-pyridinylboronic acid, Pd(dppf)Cl2, K2CO3, dioxane, H2O, 100 °C μW, 30 min (ii) TFA, 15 min; (n) B2pin2, Pd(dppf)Cl2, KOAc, dioxane, 80 °C, 3 h; (o) (i) 3-(bromomethyl)pyridine HBr, Pd(PPh3)4, K2CO3, DME, H2O, 95 °C, 10 h (ii) TFA, 30 min.