Scheme 3. Synthesis of Compounds 19, 23, 24a–f, 25, 26c, and 26f.
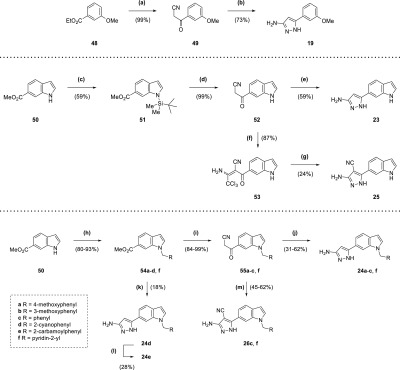
Reagents and conditions: (a) n-butyllithium (1.6 M in hexanes), acetonitrile, THF, −78 °C, 30 min; (b) N2H4·H2O, EtOH, reflux, 6 h; (c) NaH, TBDMSCl, THF, 0 °C to rt, 10 h; (d) (i) n-butyllithium (1.6 M in hexanes), acetonitrile, toluene, −78 °C, 1 h (ii) TBAF (1 M in THF), THF, 20 min; (e) N2H4·H2O, EtOH, reflux, 12 h; (f) CCl3CN, NaOAc, EtOH, 90 min; (g) N2H4·H2O, EtOH, reflux, 22 h; (h) RCH2X (·HX), Cs2CO3, acetonitrile, reflux, 1–15 h; (i) n-butyllithium (1.6 M in hexanes), acetonitrile, toluene/THF, −78 °C, 15 min to 1 h; (j) N2H4·H2O, EtOH, reflux, 5–13 h; (k) (i) n-butyllithium (1.6 M in hexanes), acetonitrile, toluene, −78 °C, 1 h (ii) N2H4·H2O, EtOH, reflux, 5 h; (l) NaOH, H2O, 100 °C, 7 h; (m) (i) CCl3CN, NaOAc, EtOH, 90 min to 9 h (ii) N2H4·H2O, EtOH, reflux, 5–15 h.
