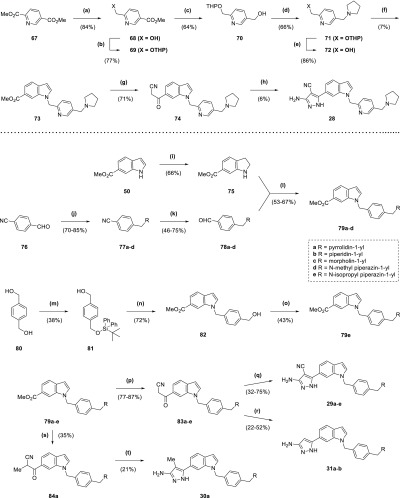
Scheme 5. Synthesis of Compounds 28, 29a–e, 30a, and 31a–b.
Reagents and conditions: (a) NaBH4, CaCl2, MeOH, THF, 0 °C, 90 min; (b) MsOH, 3,4-dihydro-2H-pyran, DCM, 150 min; (c) LiAlH4, THF, 0 °C, 45 min; (d) (i) MsCl, NEt3, DCM, 0 °C to rt, 1 h (ii) pyrrolidine, Cs2CO3, DMF, 14 h; (e) TsOH·H2O, ethanol, 50 °C, 30 min; (f) (i) MsCl, NEt3, DCM, 0 °C to rt, 150 min; (ii) 50, NaH, NaI, DMF, 0 to 60 °C, 75 min (iii) MeOH, H2SO4, reflux, 14 h; (g) n-butyllithium (1.6 M in hexanes), acetonitrile, THF, −78 °C, 1 h; (h) (i) CCl3CN, NaOAc, EtOH, 36 h (ii) N2H4·H2O, EtOH, reflux, 1 d; (i) NaCNBH3, AcOH, 0 °C to rt, 7 h; (j) Na(OAc)3BH, RH, AcOH, DCM, 80 min to 15 h; (k) DIBAL-H, THF, 0 °C to rt, 90 min to 1 h; (l) PhCO2H, toluene, 200 °C μW, 20–30 min; (m) TBDPSCl, imidazole, DMF, 21 h; (n) (i) MsCl, NEt3, DCM, 0 °C to rt, 1 h (ii) 50, NaH, DMF, 0 °C to rt, 45 min (iii) TBAF (1 M in THF), 30 min; (o) (i) MsCl, NEt3, DCM, 0 °C to rt, 90 min (ii) RH, Cs2CO3, DMF, 12 h; (p) n-butyllithium (1.6 M in hexanes), acetonitrile, THF, −78 °C, 20 min to 1 h; (q) (i) CCl3CN, NaOAc, EtOH, 4 h to 2 d (ii) N2H4·H2O, EtOH, reflux, 7 h to 1 d; (r) N2H4·H2O, EtOH, reflux, 12–21 h; (s) n-butyllithium (1.6 M in hexanes), propionitrile, toluene, −78 to 0 °C, 2 h; (t) N2H4·H2O, EtOH, reflux, 1 d.