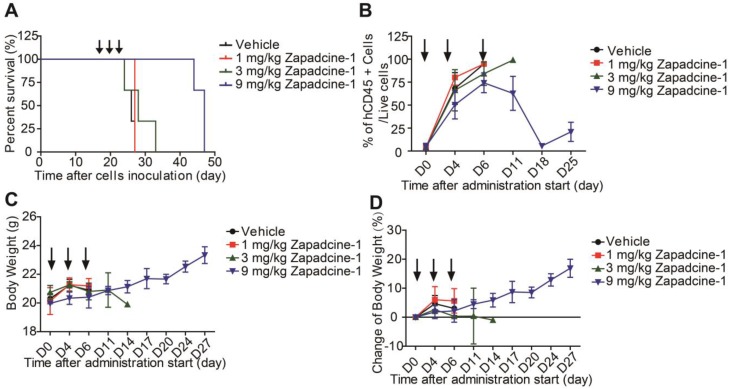
Figure 5.
Evaluation of the in vivo efficacy of Zapadcine-1 in HuKemia® acute lymphocyte leukemia PDX model AL7442. 8×105 patient AL7442 cells were intravenously injected into NOD/SCID mice for tumor development. After inoculation, blood sample were weekly collected for staining human CD45+ cells in mouse PBMC and analyzed by FACS to monitor the tumor burden. When average tumor burden reached about 4%, mice were randomly allocated into 4 groups (n = 3). The mice were intravenously injected with saline, 1.0 mg/kg, 3.0 mg/kg and 9.0 mg/kg of Zapadcine-1 on day 0, day 4 and day 7, respectively. (A) In vivo anti-tumor activity of Zapadcine-1 in HuKemia® Acute Leukemia PDX Model AL7442. Kaplan-Meier survival plots show percentage animal survival over 46 days, in which Zapadcine-1 was administered 9.0 mg/kg in comparison with vehicle. (B) Tumor burden of AL7442 mice. (C and D) Body weight and change of AL7442 mice. Q3D×3, every three days for 3 times.