Figure 1.
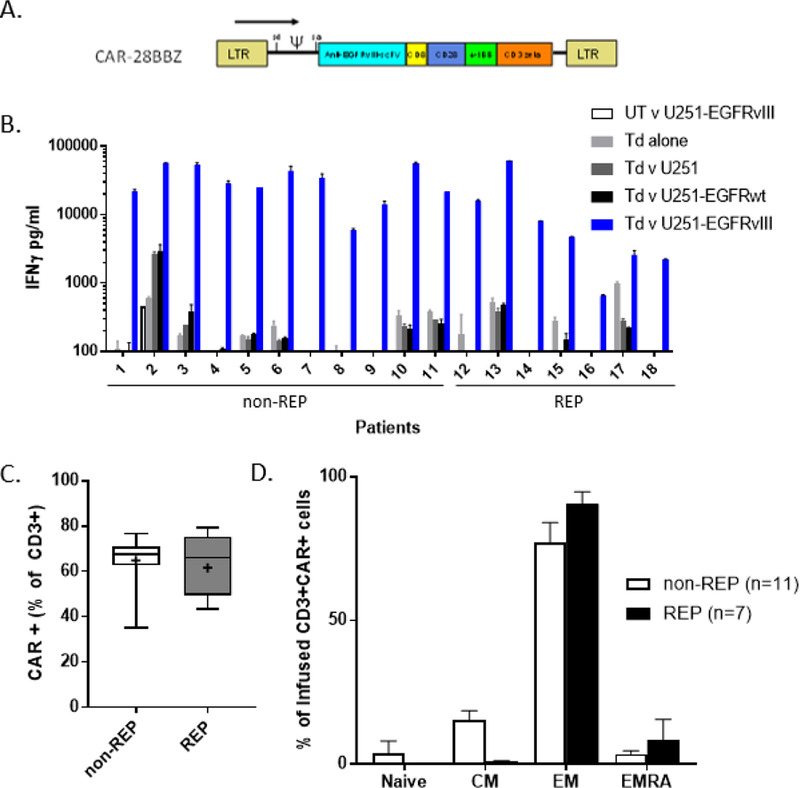
Characterization of clinical infusion product. A. Diagram of retroviral vector construct selected for clinical protocol detailing location of single chain variable fragment of human monoclonal Ab 139, CD8 linker domain, CD28 and 4–1BB costimulatory domains, and CD3ζ signaling domain. B. EGFRvIII-specific cytokine release of cell product 48–72 hours prior to infusion as measured by interferon-γ ELISA. UT: untransduced PBL, Td: PBL transduced with CAR-28BBZ, U251: glioblastoma cell line ± transduction to express EGFRwt or EGFRvIII, REP: rapid expansion protocol C. Measurement of CAR (+) cells in each infusion product by flow cytometry. Non-REP: median 67.5% (IQR 62.6–71), REP: median 66% (IQR 49.4–75.3). Gated on live, CD3+ cells. Whiskers indicate range; + denotes mean. D. Infused CAR+ cells were primarily of effector memory (EM) phenotype, as defined as CD45RA-, CCR7- T cells. There were significantly fewer central memory (CM) cells in REP products (p=0.0006).
