Figure 3.
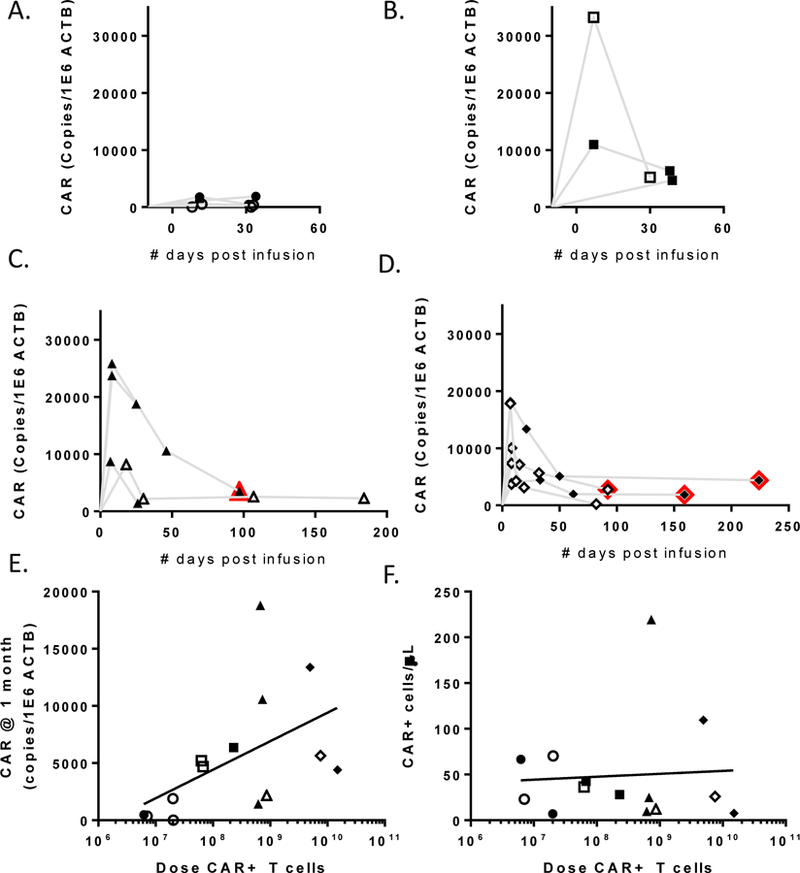
Persistence of infused CAR+ cells as measured by qPCR. A. Patients who received <3×107 CAR+ cells. B. Patients who received between 3×107 and 3×108 CAR+ cells. C. Patients who received >3×108 CAR+ cells without rapid expansion. D. Patients who received >3×108 CAR+ cells including rapid expansion. Open symbols indicate those patients receiving concurrent steroids. Red outlines indicate time points after radiologic progression. E. Persistence at one month (median day 32, n=14) was correlated with CAR+ cell dose (r=0.6, p=0.0261), but not survival (not shown). F. Persistence at one month was analyzed by FACS in conjunction with clinical lymphocyte counts, however there were too few events for reliable analysis.
