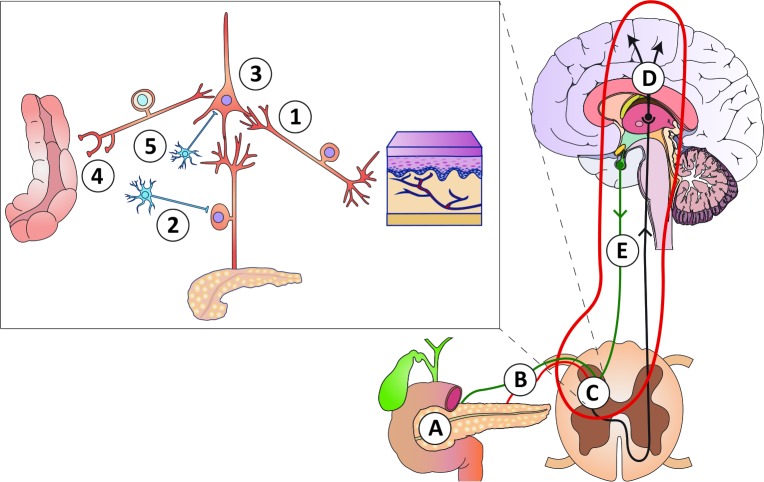
Figure 1.
Schematic overview of the pathophysiology of pain in chronic pancreatitis, for details see Olesen et al. 19 (A) Mechanical obstruction of the pancreatic duct system and/or increased intra-glandular pressure (the ‘plumbing’ theory), as well as local inflammatory masses. (B) Peripheral nerve damage with ectopic activity resulting in stimulus-dependent and spontaneous pain (the ‘wiring’ theory). (C) (insert): (1) Sprouting of non-nociceptive nerve afferents normally responsible for non-painful sensations into ’pain-specific’ areas of the spinal cord resulting in allodynia (pain to stimuli that are normally not painful); (2) sprouting of sympathetic neurons into the dorsal horn neurons rendering the system sensitive to sympathetic activity and catecholamine; (3) sensitisation and phenotypic changes of spinal neurons due to increased afferent barrage; (4) abnormal coding of the afferent input from other viscera (and somatic structures) resulting in increased referred pain and viscero-visceral hyperalgesia; (5) local disinhibition by interneurons that normally controls pain intensity. (D) Reorganisation and structural changes in the brain that encodes complex sensations such as affective, evaluative and cognitive responses to pain. (E) Defects in descending pathways arising in the brain stem that normally inhibit the peripheral afferent activity at the spinal cord level.