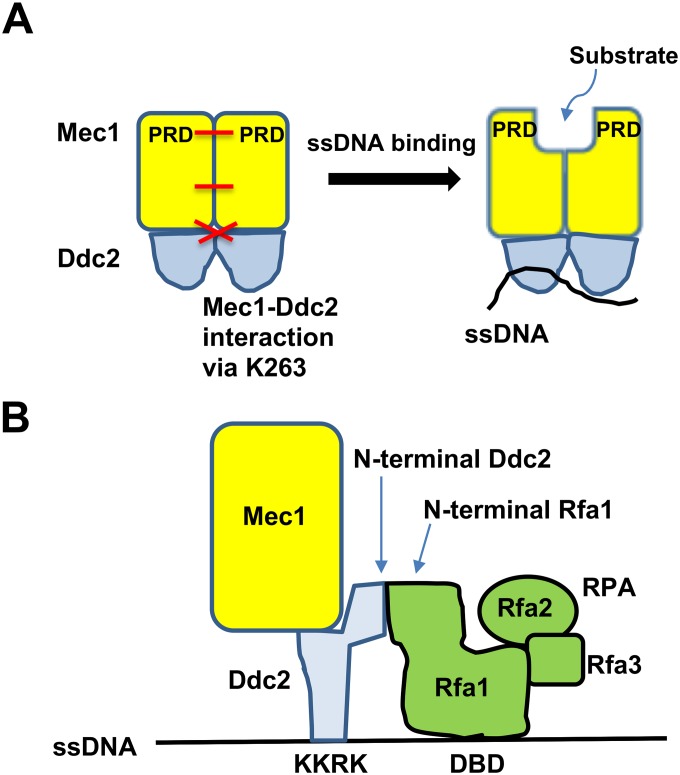
Fig 8. Model for Mec1 activation at RPA-covered ssDNA tracts.
(A) ssDNA binding of Ddc2 increases Mec1 activity. ssDNA binding of Ddc2 triggers conformation changes of the entire Mec1-Ddc2 homodimer, resulting in structural changes of the kinase domain at the C-terminus of Mec1. K263 is involved in homodimerization of the Mec1-Ddc2 heterodimer. The PRD-PRD interface within the kinase domain is also involved in homodimerization of the Mec1-Ddc2 heterodimer. See text for more detail. (B) RPA promotes ssDNA-dependent Mec1 activation. The N-terminus of Ddc2 interacts with the N-terminus of Rfa1 whereas the DNA binding (KKRK) region of Ddc2 is involved in ssDNA binding. RPA alone binds to ssDNA through its own DNA binding domain (DBD). See the text for more detail.