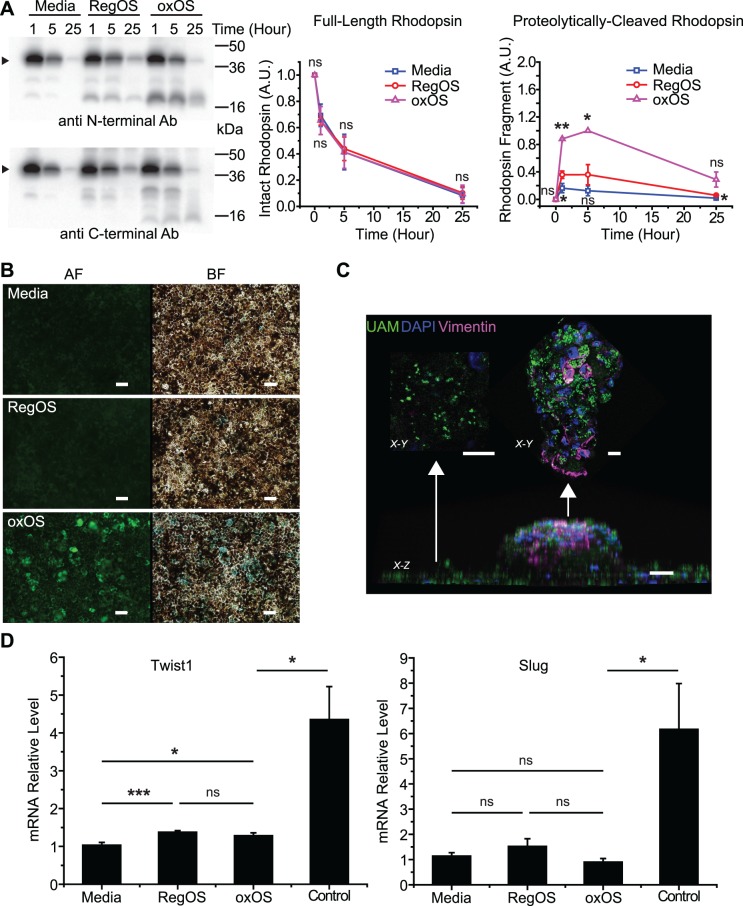
Figure 5.
UAM induces mild phenotypic effects on hfRPE cultures. (A) Consumption of an OS bolus by hfRPE cultures previously fed oxOS to form UAM (oxOS) or previously fed RegOS or media only and therefore without UAM (RegOS, media). Amount of rhodopsin remaining in the combined supernatant plus cell lysate at 1, 5, and 25 hours after initiation of bolus. No OS washoff is performed, consistent with the “pulse-only” method outlined previously.19 Arrow points to monomer band of rhodopsin. Smaller bands represent rhodopsin fragments, identified by N-terminal antibody B630 (above) and C-terminal antibody 1D4 (below). (Right) Quantification of monomer band rhodopsin shows no difference in consumption rates between conditions, but quantification of the main rhodopsin fragment recognized by B630 demonstrates higher formation rate and slower degradation of this fragment in hfRPE with UAM (oxOS); n = 3. (B) UAM-laden cultures (oxOS) have dramatically higher incidence of senescence than cultures previously fed RegOS or media only. AF, autofluorescence channel; BF, brightfield channel. Beta-galactosidase activity staining appears blue in BF channel. Scale bar: 50 μm. (C) Appearance of an “RPE clump,” a rare focal area where UAM accumulation is particularly high. X-Z view demonstrates that the clump grows out of cell monolayer, although confocal analysis shows the clump is still a single cell layer thick. X-Y view from top of clump shows intense vimentin staining, whereas X-Y view from surrounding monolayer shows minimal vimentin staining. UAM (green), nuclei (blue), vimentin (purple). Scale bar: 20 μm. (D) qPCR demonstrates minimal differences in expression of EMT markers Twist1 or Slug in cultures with UAM (oxOS) versus without (RegOS, media), in contrast to controls. Control lysates are from hfRPE plated on plastic wells (rather than Transwells) at low density, conditions that encourage EMT. n = 12 for oxOS, RegOS, media conditions, n = 5 for control condition. *P < 0.05, **P < 0.01, ***P < 0.001.