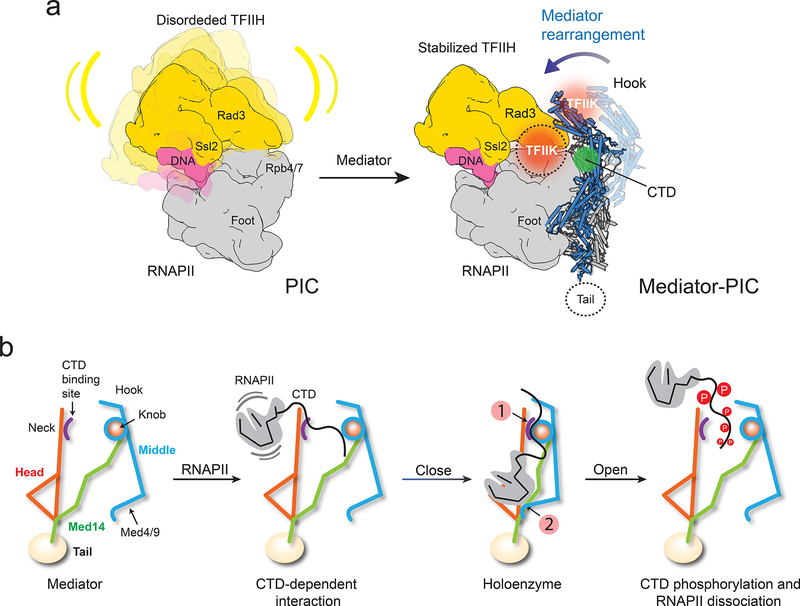
Figure 5. Mediator stabilization of the PIC and rearrangements that orchestrate RNAPII interaction.
a, TFIIH (yellow) is highly mobile in the PIC (left). Repositioning of the Middle upon holoenzyme formation (Mediator Middle in light blue, holoenzyme Middle in dark blue) would stabilize TFIIH (right) by bringing Mediator’s hook into direct contact with TFIIH’s Rad3 and TFIIK. TFIIH stabilization would explain enhancement of basal transcription and CTD phosphorylation by Mediator. A cryo-EM map of the PIC and TFIIH 2D class averages show TFIIK as indicated (orange circle). An alternate TFIIK position on the opposite side of the hook (faded orange circle) observed in some TFIIH 2D averages could be favored upon Mediator interaction. Either placement would put TFIIK immediately adjacent to its CTD target (CTD position indicated by green circle). b, CTD binding at a site accessible in free Mediator would initiate RNAPII interaction, help trigger a change to the holoenzyme conformation (with a closed CTD-binding gap and the CTD engaged between the Head’s neck and the knob (indicated by number 1 in a red circle), enable a Mediator-Rpb1 foot contact (indicated by number 2 in a red circle), stabilize RNAPII/PIC interaction and position the CTD for phosphorylation by TFIIK. After initiation, CTD phosphorylation by TFIIK would help revert Mediator to its free conformation, with an open CTD-binding gap and movement of Med4/9 away from Rpb1. This would disrupt Mediator interaction with RNAPII/PIC and facilitate the transition to elongation.