Abstract
Engaging a telomere maintenance mechanism during DNA replication is essential for almost all advanced cancers. The conversion from normal and premalignant somatic cells to advanced malignant cells often results (85–90%) from the reactivation of the functional ribonucleoprotein holoenzyme complex, referred to as telomerase. Modulation of the human telomerase reverse transcriptase (hTERT) appears to be rate limiting to produce functional telomerase and engage a telomere maintenance mechanism. The remaining 10–15% of cancers overcome progressively shortened telomeres by activating an alternative lengthening of telomeres (ALT) maintenance mechanism, through a DNA recombination pathway. Exploration into the specific mechanisms of telomere maintenance in cancer have led to the development of drugs such as Imetelstat (GRN163L), BIBR1532, 6-thio-dG, VE-822, and NVP-BEZ235 being investigated as therapeutic approaches for treating telomerase and ALT tumors. The successful use of 6-thio-dG (a nucleoside preferentially recognized by telomerase) that targets and uncaps telomeres in telomerase positive but not normal telomerase silent cells has recently shown impressive effects on multiple types of cancer. For example, 6-thio-dG overcomes therapy-resistant cancers in a fast-acting mechanism potentially providing an alternative or additional route of treatment for cancer patients. In this perspective, we provide a synopsis of the current landscape of telomeres and telomerase processing in cancer development and how this new knowledge may improve outcomes for cancer patients.
Keywords: ALT, BRAF, hTERT, hTERC, melanoma, telomerase, 6-thio-dG
1. Background
Recent advances in developing therapies for treating patients with advanced cancers have significantly increased patients’ overall survival. Targeted therapies and cancer immunotherapies have provided advanced cancer patients with hopes of durable remissions that are supplementing or replacing standard chemotherapy and radiation therapy. Many cancers, however, inevitably develop resistance to drug and radiation regimens and eventually re-establish the tumor ecosystem following initial responses. In order to improve durable long-lasting responses and overall patient survival rates, numerous efforts have been directed toward understanding molecular mechanisms by which tumors exhibit intrinsic resistance or develop acquired resistance to existing therapies. One of the more recent areas of interest includes the modulation of telomeres, due to their key function in the maintenance of cancer cells.
Telomeres, the end caps of eukaryotic linear chromosomes (Figure 1), have a major physiological role important in the overall proliferative lifespan of somatic cells. Telomeres act as guards during cell replication, and function to protect cells from being recognized as DNA damage. Mammalian telomeres consist of d-TTAGGG repeats and contain a specific “shelterin” complex of six proteins, which prevents end-to-end chromosomal fusions and recognition as DNA damage.1 They are essential for chromosomal maintenance and genomic stability.2 Telomeres shorten when normal cells undergo each replication due to the “end replication problem”.3 This leads to a natural senescent phenotype once a specific short length, often termed the “Hayflick limit”, is reached.4 This process is largely involved in the aging process of normal cells due to a lack of engaging a telomere lengthening mechanism.
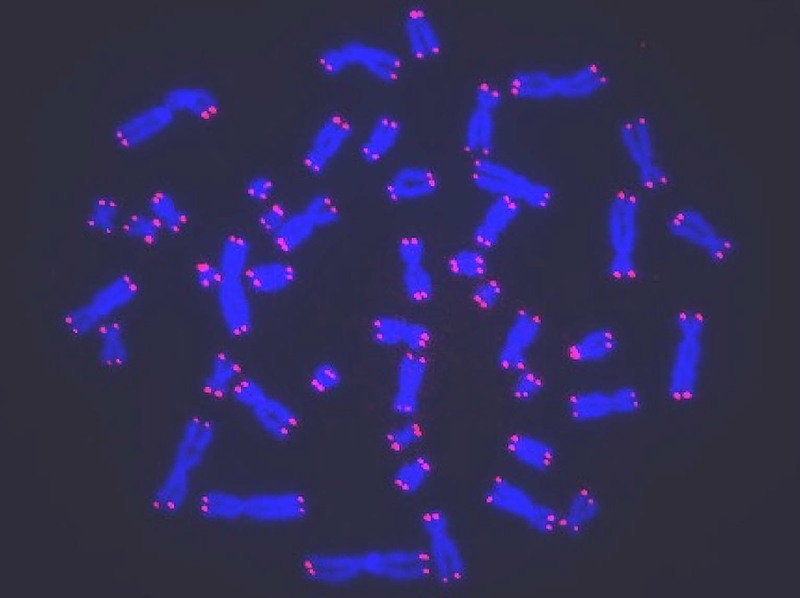
Figure 1. Visualization of human telomeres on metaphase chromosomes using digital fluorescence microscopy.
Human cells were treated with colcemid to arrest cells in mitosis and chromosome spreads were made. Samples were prepared for quantitative fluorescence in situ hybridization (Q-FISH) microscopy using labeled peptide nucleic acid probes specific for (TTAGGG)n telomere sequences (red color) and the general DNA dye DAPI (blue color). Fluorescent images were acquired on a digital imaging microscope system to calculate the fluorescence intensity for each telomere. The telomere length is proportional to the number of hybridized probes.
Some proliferating, stem-like cells and cancer cells engage a functional ribonucleoprotein enzyme complex termed telomerase that works to counteract the telomere shortening problem by adding telomeric DNA repeats to the ends of the chromosomes.4 Telomerase, itself, contains two major components. The first, human telomerase reverse transcriptase (hTERT) embodies the catalytic reverse transcriptase protein component of the holoenzyme. In combination with other components of telomerase, human TERT helps to modulate cell survival and proliferation through telomerase-dependent telomere lengthening.5 Human TERT also acts in a telomerase-independent fashion through intermolecular interactions, specifically involving TP53 (p53) and poly(ADP-ribose) polymerase (PARP).6 For example, down regulating hTERT expression in breast cancer-induced cell death in a telomere length-independent fashion is counteracted by upregulating p53, demonstrating a potential connection between the two. Secondly, the human telomerase RNA component (hTERC) serves as the functional RNA component, which works through its template region to direct the synthesis of the correct 5’–TTAGGG repeats for addition to the 3’ telomere end. Telomerase is inactive in most but not all somatic cells, while it is active in 85–90% of cancer cells.7,8,9 This allows telomerase to act as a biomarker to differentiate cancer cells from almost all somatic cells, while also providing a possible avenue of cellular survival and proliferation control. In somatic cells that proliferate frequently (e.g. skin, intestines and blood), telomerase can be transiently activated increasing the number of divisions a normal cell can undergo. However, this transient activation of telomerase becomes silenced upon differentiation. Little is known about the mechanisms that regulate telomerase in these highly proliferative somatic cells. In contrast, cancer cells appear to maintain telomerase activity to control telomere length in order to continuing dividing. However, the elongation of telomeres by telomerase is not the only method by which this process can occur. Additional mechanisms that regulate the alternative lengthening of telomeres (ALTs) maintenance pathway provides soft tissue sarcomas, some gliomas and a minority of other cancer cell types with a second method to maintain the lengthening behavior.
2. Telomerase
Telomerase, which is normally silent or present at a very low level in somatic cells, can be reactivated in the process of transforming normal cells to cancerous cells.10 It is hypothesized that telomerase can be reactivated in a variety of ways, though the precise mechanisms by which it is activated are still largely unknown. Often though, telomerase can be activated due to mutations in the non-coding promoter hTERT region. Indeed, telomerase promoter mutations are believed to be the most common non-coding mutation in cancer.11 Historically, protein-coding regions of genes or splice junctions were the only known somatic mutations in tumors due to their high prevalence.12 In 2013, however, mutations in hTERT promoter regions were observed in a wide variety of cancers.12,13 This observation altered the mutational landscape, leading to further investigation into regulatory mutations and their significance in relation to carcinogenesis.
2.1. Mutations in hTERT Promoter Regions
Point mutations in the hTERT promoter - specifically, C228T and C250T - were first observed in familial malignant melanomas that did not carry germline mutations.12,13 Mutational loads appear to correlate with telomere length and with the age of the cell. There is mounting evidence supporting the idea for a telomere chromatin loop structure in the hTERT promoter region (~1 Mb from the telomere) that is conserved in young cells with elongated telomeres.14 This occurs in most large long-lived mammals and with progressive telomere shortening this telomere looping structure is lost, changing the epigenetic landscape in the locus around the TERT gene facilitating promoter mutations14. Moreover, shorter telomeres have been linked to increased genomic alterations, and may possibly explain the regular occurrence of hTERT promoter mutations during periods of decreased telomere length.15 C228T and C250T point mutations increase transcriptional activity of hTERT through the creation of a new binding motif for ETs transcription factors and ternary complex factors (TCFs) near the transcriptional start of hTERT.13
A high prevalence of mutations in the same hTERT promoter region were observed in sporadic melanomas, which also led to increased transcriptional rates. The cytidine-to-thymidine point mutations (C228T and C250T) were observed within 100 base pairs of the hTERT transcriptional start and appeared mutually exclusive. These studies demonstrated an ability to increase the transcriptional activity from the hTERT promoter when this region is mutated.12,13,16 The frequency of mutations in the hTERT promoter region was higher than that of either BRAF or NRAS mutations in melanomas, suggesting hTERT as an important factor in inducing carcinogenesis through its dysregulation.12,13 Furthermore, tumors with BRAF or NRAS mutations contained hTERT promoter mutations at a statistically significant higher rate than those without mutations, suggesting that BRAF or NRAS mutations accelerate the melanomagenesis in concert with hTERT promoter mutations.17 It has also been shown that hTERT promoter mutations could be used as an independent prognostic factor in cutaneous melanomas.17
In 2015, Shain and colleagues sought to explore the evolutionary trajectory of 37 melanocytic neoplasms, in order to provide a depiction of the genetic order of acquisition of specific oncogenic mutations.18 Those 37 melanocytic neoplasms were grouped into four histologic categories including benign lesions, intermediate lesions, melanoma in situ, and invasive melanomas. hTERT promoter mutations were present in 77% of melanomas that were classified as either intermediate lesions or melanoma in situ.18 All neoplasms were thought to have been initiated from mutations activating the MAPK pathway, with variations in hTERT being the first set of secondary mutations observed in intermediate lesions. The observation that hTERT promoter region mutations occur early on in cancer development has also been revealed in a study of bladder cancer.19 The presence of hTERT mutations appears to explain the cellular trajectory into melanoma, and a further mutational load once the initial MAPK pathway had been activated by oncogenic BRAF or NRAS mutations.18 This was due to the observation that in the absence of hTERT promoter mutations, most cells were incapable of surviving through replicative stressors (Figure 2). Without hTERT promoter mutations, proliferation led to mitotic errors, resulting in cell death or premature senescence.18 Shain et al. (2015) portray the evolution of benign lesions to metastatic melanomas as starting with the activation of the mitogen activated protein kinase (MAPK) pathway followed by the acquisition of hTERT promoter region mutations.18 These promoter mutations then lead to a larger and more diverse mutational load that branches the evolutionary pathway (Figure 2). Accompanying the progression of melanoma from early to a more advanced stage, further factors, such as the level of UV damage, appear to participate in the evolutionary pathway of this disease.18
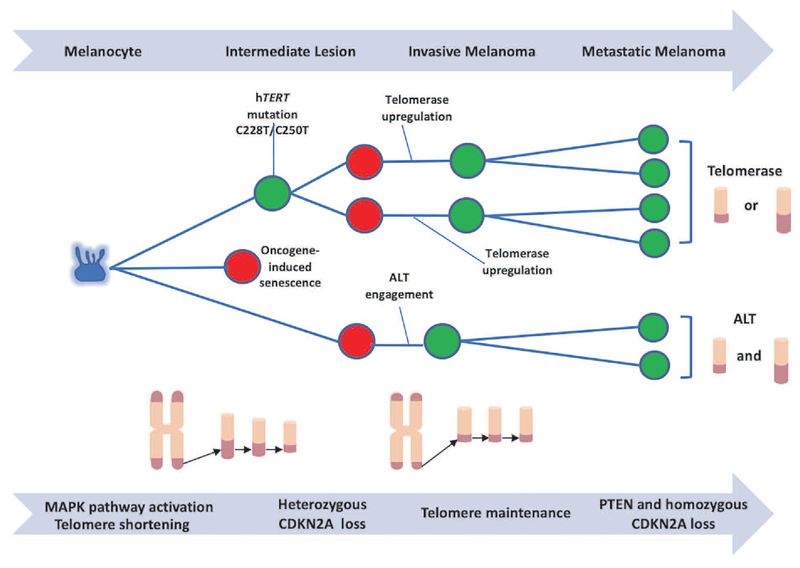
Figure 2. Description of the linear-to-branched evolutionary process of melanomas.
Melanocytes (blue) with MAPK pathway activation, due to mutations such as BRAFV600E or NRAS, proliferate and one of three things can occur. First, cells experience oncogene-induced premature replicative senescence (red) due to overexpression of the BRAF or NRAS oncogene.58 Alternatively, cells can engage a DNA recombination mechanism, termed alternative lengthening of telomeres (ALT). These cells continue dividing (green) until telomere based replicative senescence is engaged and then bypassed. Cells then enter a period called crisis. Only a rare cell can emerge from this crisis state, and ALT cells are characterized by having both long and short telomeres, ALT associated PML bodies and extra chromosomal telomere repeats (as identified by the C-circle assay). The final scenario, cells can either spontaneously upregulate telomerase or accumulate telomerase promoter mutations, allowing replication, and partially extending the proliferative life span of the cells until they reach crisis where genomic instability is increased. Then, in combination with other alterations, cells upregulate telomerase further to maintain short telomeres, but in some cases, telomeres may become longer. Once cells progress past this vital barrier and short telomere lengths are maintained, genomic stability is also maintained. However, the immortalized cells have extended time to increase the mutational load, with CDKN2A and PTEN alterations being highly prevalent, branching the evolutionary pathway even further to metastatic disease.
The direct contribution of hTERT promoter mutations towards the development of cancer is still somewhat understudied. Recently though, Chiba and colleagues asserted the idea that hTERT promoter mutations work in a two-phase mechanism leading to melanomagenesis.20 The group showed that during the transformation of four samples with hTERT promoter mutations from nevi to melanomas, telomeres continued to shorten until a crisis level was achieved. This was characterized by a critical length of telomeres that led to chromosome end-to-end fusions and death.20 This signaled that hTERT promoter mutations, leading to increased telomerase activity, were insufficient to prevent telomere shortening, and instead worked to postpone telomere-based replicative senescence for those cells with the shortest telomeres. Once telomeres had uniformly shortened beyond this extended lifespan period, pressure for further telomerase activity increased, leading to cell immortalization.20 In other words, the promoter mutations initially bypassed the initial DNA damage signal from a few short telomeres until telomere lengths of most chromosome ends were short enough to induce crisis (a period of balance between cell growth and death). Then, due to pressures occurring at the crisis stage most cells die, but telomerase is selectively further upregulated to prevent cell death albeit only in a rare subset of cells. Here, Chiba and colleagues point to the routine shortening of telomeres as a necessary barrier to overcome for melanoma tumorigenesis to occur.20
Separately, hTERT promoter mutations do not seem to be confined to melanomas, as evidenced by Huang and colleague’s observation of the existence of hTERT promoter mutations in samples of bladder and hepatocellular cancer cell lines.12 Again in 2013, a more widespread analysis of a plethora of tumors was conducted in which this group analyzed 1,230 tumor samples representing 60 different tumor types.21 They found that 18.8% of the samples analyzed contained hTERT promoter mutations, with 98.3% of those mutations being C250T or C228T.21 Samples were then categorized by the prevalence of hTERT promoter mutations in order to identify which types of normal cells were more likely to be transformed into cancer with the contribution from the hTERT promoter mutations. The results were that hTERT promoter mutations are significantly more common in cells that do not continually self-renew.21 This point was emphasized and supported by the fact that hTERT promoter mutations were rare in pediatric primary glioblastoma multiforme (GBM), while being extremely common in adult GBM.21 These findings further cemented the relationship between the self-renewal capacity of somatic cells and the likelihood of hTERT promoter mutations contributing to their carcinogenesis. Vinagre et al. further added support to these findings, reporting a comparison between hTERT promoter region mutations in self-renewing and non-self-renewing cancers.22 This group also added to the repository of cancer types with hTERT promoter mutations by reporting their observations in follicular cell-derived forms of thyroid cancer for the first time.
In addition, mutations in the hTERT promoter region are also associated with the invasive potential of various cancers and specifically distant metastases.23 In a study of ureter carcinomas, it was found that 12.5% of patients with hTERT promoter region mutations developed metastatic disease, while patients without mutations rarely (1.3%) developed metastases.23 For melanomas, Horn et al. and Griewank et al. have found notable differences in the frequency of point mutations in the hTERT promoter region between primary (33–37%) and metastatic tumors (50–85%), demonstrating a correlative role for hTERT in increasing the metastasis potential of melanomas.13,17 Furthermore, the presence of hTERT mutations in a study of 327 patients with urothelial cell carcinoma of the bladder showed a hazard ratio of 1.34 for the overall effect of the mutation on the patient.19 Poor survival prognosis associated with hTERT promoter mutations has also been described by Killela et al. with specific regard to gliomas.21
2.2. hTERT Inhibition
Inhibition of telomerase through genetically depleting hTERT or therapeutically targeting telomerase has become more attractive recently due to the correlation hTERT promoter mutations with the proliferation of cancer cells.10,12,13,16 It has also been hypothesized that hTERT plays a specific role in modulating NF-kappaB, TGF-beta/Smad, and Wnt signaling pathways in cancer cells.24,25,26 In the past though, efforts to therapeutically inhibit telomerase proved to be of marginal clinical utility due to lengthy treatment times and increases in hematological toxicities prior to the onset of any benefit.
It was shown that GRN163L, a 13-mer thiophosphoramidate oligonucleotide also referred to as Imetelstat, was able to inhibit telomerase in a dose-dependent manner through competitive inhibition.27 GRN163L induced growth arrest, as well as telomere shortening, in multiple cancer cell lines.8,27,28 However, GRN163L lacks a suitable time-related efficacy due to it necessitating multiple replication processes of the telomeres in order for it to effectively drive telomeres to critically short lengths and cell death.8,28 In other words, the length of time required to shorten the telomere length significantly affected the usage of GRN163L in a clinical setting, and has rendered the drug most useful in cases where telomeres are already shortened.28 Studies on advanced non small cell lung cancer patients showed only limited efficacy29, and in pediatric brain cancer patients GRN163L (Imetelstat) was only able to be administered for an average of 13 days (a 6-to-21-day range) prior to intolerable toxicity levels characterized by platelet nadir being established.30 Moreover, GRN163L drug holidays, due to these increased toxicities, lead to rapid reestablishment of telomere length and continuing cell growth.27 For instance, it was reported that 2 weeks after termination of long-term GRN163L administration, A549-Luc cells were able to reform colonies at an equivalent rate as prior to drug treatment.27
Telomerase was also identified as an enzyme that is very similar to the reverse transcriptase of viruses at both the structural and mechanistic levels, thereby eliciting the hypothesis that inhibitors of reverse transcriptase may work to inhibit telomerase.31 BIBR1532, a non-nucleotidic synthetic small-molecule drug, was identified as a selective inhibitor of telomerase.31 In 2002, it was demonstrated that BIBR1532 acted as a mixed-type non-competitive inhibitor, and specifically interacted with the hydrophobic pocket of the thumb domain of telomerase, thereby reducing the number of hexameric repeats that could be added.30,32 This synthetic small molecule was later shown to contribute to the success of chemotherapy agents through the above mechanism.30,33,34 Most recently, BIBR1532 has been shown to induce apoptosis amongst various breast cancer cell lines.36,37 While the molecular mechanism of BIBR1532 inhibition remains to be elucidated in detail, it has been shown that BIBR1532 suppresses survivin and further activates apoptotic-associated factors such as p73, Bax/Bcl‐2, and caspase-3.38 However, BIBR1532 does not appear to be in clinical development at the present time and is likely to have the same tissue toxicities issues as GRN163L.
Thiopurine-induced inhibition research has provided an alternate route of telomere control in cancer cells expressing telomerase. Historically, thiopurines have been used for the treatment of leukemia, as well as in immunosuppression.39 Their uses for cancer treatment has generally been confined to leukemia and some pediatric cancers due to the high toxicity levels exhibited in patients treated with thiopurines.8 Thiopurines undergo metabolic activation reactions that eventually synthesize the molecule, 6-thio-2’-deoxyguanosine-5’-triphosphate. This molecule is then incorporated into DNA strands during replication, leading to rapid uncapping of telomeres and cell death.8
Since telomerase was known to have a high affinity for guanine bases containing 2’-deoxyguanosine-5’-triphosphate, it was hypothesized that a designed analogue of 6-thioguanine may more rapidly use the enzyme to iincorporate an alter guanine in the telomere while limiting toxicity in telomerase silent normal cells. By incorporating 6-thio-2’-deoxyguanosine 5’-triphosphate into the telomeric DNA, damages caused to DNA and proteins can occur, thereby leading to the failure of replication and the uncapping of telomeres. This effect has been termed telomere dysfunction-induced foci (TIFs), and leads to rapid senescence and/or apoptosis but only in telomerase expressing cells.8 It was shown that a thiopurine analog, 6-thio-dG, successfully led to the rapid uncapping of telomeres.8 Here, 6-thio-dG was found to be less toxic compared to equal molar equivalents of 6-thioguanine.8 Incorporating 6-thio-2’-deoxyguanosine 5’-triphosphate into DNA replaces the –GGG- repeats with 6-thio groups, thereby altering the biochemistry of the telomere structure. This change is believed to sequentially alter the protective shelterin protein complex, and thus induces TIFs (as identified by co-localization of a shelterin protein with a DNA damage antibody such as 53BP1 or gamma-H2AX). 6-thio-dG also decreased the lag period experienced by previous direct telomerase inhibitors and demonstrated an independence of telomere length in its ability to affect the cancer in a timely manner.8 Importantly, 6-thio-dG did not have a significance effect on normal telomerase silent cells. 6-thio-dG has been shown to provide a unique method of targeting the otherwise “untargetable” NRAS and RAS oncogene-induced cancers.40 Being that NRAS mutations are the second most frequent mutations occurring in melanoma41,42, the improved results with administration of 6-thio-dG in combination with Gamitrinib should provide hope for the future of widespread usage of the drug.40
Even more recently, 6-thio-dG was shown to successfully prolong disease control in pre-clinical models of melanoma that acquired resistance to targeted therapies (e.g. BRAF inhibitors) or immunotherapies.43 6-thio-dG, was analyzed in combination with targeted therapies and as a monotherapy. When used as a monotherapy, 6-thio-dG outperformed BIBR1532 in terms of its anti-proliferative ability and did not result in any significant hematological or hepatotoxicities.43 Moreover, the inhibitory effect of 6-thio-dG was comparable to that of the notable BRAF inhibitor, PLX4720, and impaired tumor growth in xenograft models in a comparable manner to another BRAF inhibitor, dabrafenib.43 Even more importantly, 6-thio-dG appears to tackle the issues of acquired resistance and lack of efficacy for immune checkpoint blockade inhibitors and targeted therapies. Lately, 6-thio-dG exhibited an effective ability to overcome EGFR targeted- and platin-doublet chemotherapy resistance in NSCLC, as well as in therapy-resistant pediatric brain cancer.44 While targeted therapies have significantly improved the options available for unresectable or metastatic cancers, relapse almost always occurs through a variety of pathways that mediate acquired resistance. Thus, it is hopeful that 6-thio-dG will work as a front line or salvage therapy towards targeting therapy-resistant cancer cells and may sensitize tumors that are refractory to checkpoint inhibitors providing long-term durable responses. In a preclinical study on therapy-resistant pediatric brain tumors, it was also demonstrated that 6-thio-dG can cross the blood brain barrier thus expanding the utility of this new approach.45
2.3. hTERC Inhibition
In parallel, recent studies have also revealed that hTERC (the functional RNA component of the telomerase holoenzyme) plays an important role in cancer development. While the specific contribution of hTERC is largely unknown, it was previously noted that suppression of hTERC in a large panel of cancer cells including lymphoma, melanoma, bladder, breast, and colorectal carcinoma inhibited growth and led to apoptosis.46,47,48 More specifically, hTERC has been shown to be over-expressed in all phases of prostate carcinogenesis, as well as linked to the oncogene, MYC.49 Specifically, when MYC was reduced hTERC levels temporarily decreased, while during MYC overexpression, hTERC levels increased.49 This correlation was not limited to prostate carcinogenesis, and instead was replicated in the same study using a non-small cell carcinoma cell line (NCI-H23), a breast cancer cell line (MCF-7), and a colorectal carcinoma cell line (DLD-1).49 Again, the mechanism is not fully understood albeit the knock-down of hTERC limited telomerase activity, highlighting its importance in immortalizing cells via telomere maintenance and elongation pathways. Even more recently though, over-expression of hTERC was shown to occur in tumors compared to the normal tissues suggesting that hTERC was involved in some other cellular functions beyond telomerase.50 Furthermore, it was also shown that over-expression of hTERC can contribute to cell apoptosis, in a separate fashion from its function in telomerase.46 The exact role hTERC plays outside of the telomerase complex is still largely unknown and warrants further investigation, as it may provide alternative routes of modulating cellular immortalization.51
3. Alternative Lengthening of Telomeres
In order for cancer cells to maintain their proliferative phenotype and malignant nature, they must overcome telomere shortening during the large number of replications required to accumulate sufficient mutations to become malignant. Treating cancer cells that express telomerase with inhibitors can potentially elicit the phenotype of alternative lengthening of telomeres (ALT) which is telomerase-independent.52 Cells are capable of adapting to the telomerase inhibitors, leading to upregulation of more telomerase (common) or the activation of alternative lengthening of telomeres (much less common) in a telomerase-independent fashion.7,52–56 In a comprehensive analysis of a large panel of primary tumors representing multiple different cancer subtypes, only 3.74% presented with a positive ALT phenotype.56 Presence of the ALT phenotype did depend on the cancer subtype, however, with a greater prevalence being observed in sarcomas (25–60%) and 5–15% in carcinomas.56 Conversely, the ALT phenotype was largely absent in urothelial carcinomas, gastric carcinomas, and adenocarcinomas of most major cancer types, accentuating the idea that ALT is more common in mesenchymal and neuroepithelial derived tumor types.52–56 Overall, it is believed that 5–15% of cancer cells lack telomerase activity and are maintained by ALT.10,52–56 Regardless of the mechanism by which ALT is engaged, ALT-dependent cells rely on genetic recombination to continuously elongate the telomeres.52,54 Since ALT-dependent cells are homologous recombination (HR) proficient, while many other cancer cells are not, this provides an avenue for the exploration of ALT-based cancer treatments. Differences that distinguish ALT-dependent cells from telomerase-positive cancer cells include longer telomere overhangs and preferentially elongated lagging strands depicted in S phase during replication, meaning that the length of telomeres in ALT-positive cancer cells are heterogeneous and vary greatly in length.52 Recently, telomerase-positive H1299 and SW39 telomerase positive cell lines have been modulated using the CRISPR/Cas9 technique to knockout hTERC in an attempt to activate the ALT pathway.54 It was shown that upon the depletion of hTERC, a very small percentage of cells were able to survive, and those that did all displayed elongated telomeres of varying lengths and the presence of ECTR (extra chromosomal telomere repeats) as determined by the C-circle assay.54 It is speculated that those methods by which ALT is acquired in cells are affected by the degree of the depletion of ATRX or DAXX and hTERC.54, 55 In this case, the adoption of the ALT pathway is in general, a seemingly “last resort” adaptation in response to adverse events that would otherwise kill the cells. While the direct molecular mechanisms underlying the action and initiation of ALT are still largely unknown, some methods of ALT inhibition have been explored. ATR inhibitors, such as VE-822 and NVP-BEZ235 have been shown to more selectively target and kill cells that are positive for ALT.53
4. Conclusions
Telomerase-mediated modulation of telomere dynamics continues to be a promising area of investigation in regard to the therapeutic control of tumors. Telomeres naturally shorten in somatic cells over gradual divisions, while in cancer cells their lengths are maintained in order to ensure continuous proliferation. This provides a future direction and platform to therapeutically explore approaches to identify and exploit telomerase, an almost universal cancer vulnerability. Telomerase, being the major mode of telomere lengthening in cancer cells has been explored with many recent advances taking place. Historically, targeting telomerase was ineffective in the clinical setting due to the long lag period from initiation of treatment until effect (e.g. many cellular replication cycles had to take place in order to achieve the benefit from the inhibition). Recently, however, advances have occurred with the exploration of 6-thio-dG, a small molecule that effectively reduces the lag time observed with direct telomerase inhibitors as well as the toxicities usually occurring with thiopurine molecules. While ALT-positive cancer cells are relatively rare, they are beginning to be examined as clinical cancer targets. In conclusion, the importance of achieving full control over telomere length is becoming clearer leading to the potential for modulation to be effective in a high percentage of cancer patients. Due to the universal relationship between cancer and telomere maintenance there is a clear promise for the future.
Acknowledgments
Supported by AG01228 from the National Institute on Aging and NCI SPORE P50CA70907. We also acknowledge the Harold Simmons NCI Designated Comprehensive Cancer Center Support Grant (CA142543), and the Southland Financial Corporation Distinguished Chair in Geriatric Research. This work was performed in laboratories constructed with support from NIH grant C06 RR30414.
List of Abbreviations
- hTERT
Human telomere reverse transcriptase
- hTERC
Human telomerase RNA component (also referred to as hTR)
- CDKN2A
cyclin-dependent kinase Inhibitor 2A; gene involved in tumor suppression (also referred to as p16 or p16INKa)
- BRAF
Gene involved in cell signaling that is often mutated in melanoma
- NRAS
Gene involved in cell signaling and the regulation of cell division
- ALT
Alternative lengthening of telomeres (telomerase independent)
- TIFs
Telomere dysfunction-Induced Foci
- 6-thio-dG
6-thio-2’-deoxyguanosine
- PARP
poly(ADP-ribose) polymerase
- MAPK
mitogen-activated protein kinase
References
- 1.Shay JW, Reddel RR, & Wright WE (2012). Cancer and telomeres—an ALTernative to telomerase. Science, 336(6087), 1388–1390. [DOI] [PubMed] [Google Scholar]
- 2.Naito T, Matsuura A, & Ishikawa F (1998). Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nature genetics, 20(2), 203. [DOI] [PubMed] [Google Scholar]
- 3.Watson J Nature New Biology (1972) 239: 197. [DOI] [PubMed] [Google Scholar]
- 4.Shay J, & Wright W (2011). Role of telomeres and telomerase in cancer. Seminars in Cancer Biology., 21(6), 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y, Zhang Y, Zhang Z, Miao Z, & Ding J (2008). hTERT-targeted RNA interference inhibits tumorigenicity and motility of HCT116 cells. Cancer Biology & Therapy., 7(2), 228–236. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Li H, Deb S, & Liu J (2002). TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene., 21(20), 3130–3138. [DOI] [PubMed] [Google Scholar]
- 7.Shay J, & Bacchetti S (1997). A survey of telomerase activity in human cancer. European Journal of Cancer., 33(5), 787–791. [DOI] [PubMed] [Google Scholar]
- 8.Mender I, Gryaznov S, Dikmen Z, Wright W, & Shay J (2015). Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2’-deoxyguanosine. Cancer Discovery., 5(1), 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shay J, & Wright W (2001). Telomeres and Telomerase: Implications for Cancer and Aging. Radiation Research., 155(1), 188–193. [DOI] [PubMed] [Google Scholar]
- 10.Corey D (2009). Telomeres and telomerase: From discovery to clinical trials. Chemistry & Biology., 16(12), 1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zehir A, Benayed R, Shah R, Syed A, Middha S, Kim H, … Mandelker D (2021). Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature Medicine., 23(6), 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang F, Hodis E, Xu M, Kryukov G, Chin L, & Garraway L (2013). Highly recurrent TERT promoter mutations in human melanoma. Science., 339(6122), 957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn S, Figl A, Rachakonda P, Fischer C, Sucker A, Gast A, … Kumar R (2013). TERT promoter mutations in familial and sporadic melanoma. Science., 339(6122), 959–961. [DOI] [PubMed] [Google Scholar]
- 14.Kim W, Ludlow A, Min J, Robin J, Stadler G, Mender I, … Durocher D (2016). Regulation of the human telomerase gene TERT by telomere position effect—over long distances (TPE-OLD): Implications for Aging and Cancer. PLoS Biology., 14(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos G, Kröber A, Grabowski P, Kienle D, Bühler A, Döhner H, … Stilgenbauer S (2008). Short telomeres are associated with genetic complexity, high-risk genomic aberrations, and short survival in chronic lymphocytic leukemia. Blood., 111(4), 2246–2252. [DOI] [PubMed] [Google Scholar]
- 16.Akbani R, Akdemir K, Aksoy B, Albert M, Ally A, Amin S, … Belyaev D (2015). Genomic classification of cutaneous melanoma. Cell., 161(7), 1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griewank K, Murali R, Puig-Butille J, Schilling B, Livingstone E, Potrony M, … Schadendorf D (2014). TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. Journal of the National Cancer Institute: JNCI., 106(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shain A, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, … Bastian B (2015). The Genetic evolution of melanoma from precursor lesions. The New England Journal of Medicine., 373(20), 1926–1936 [DOI] [PubMed] [Google Scholar]
- 19.Rachakonda P, Hosen I, de Verdier P, Fallah M, Heidenreich B, Ryk C, … Kumar R (2013). TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proceedings of the National Academy of Sciences of the United States of America., 110(43), 17426–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiba K, Lorbeer F, Shain A, McSwiggen D, Schruf E, Oh A, … Hockemeyer D (2017). Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science., 357(6358), 1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killela P, Reitman Z, Jiao Y, Bettegowda C, Agrawal N, Diaz L, … Riggins G (2013). TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proceedings of the National Academy of Sciences of the United States of America., 110(15), 6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinagre J, Almeida A, Pópulo H, Batista R, Lyra J, Pinto V, … Cameselle-Teijeiro J (2013). Frequency of TERT promoter mutations in human cancers. Nature Communications., 4:2185 doi: 10.1038/ncomm3185. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Liu T, Ge N, Liu L, Yuan X, Liu J, … & Hu S (2014). TERT promoter mutations are associated with distant metastases in upper tract urothelial carcinomas and serve as urinary biomarkers detected by a sensitive castPCR. Oncotarget, 5(23), 12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Bian C, Zhen C, Liu L, Lin Z, Nisar MF, … & Yang L (2017). Telomerase reverse transcriptase mediates EMT through NF-κB signaling in tongue squamous cell carcinoma. Oncotarget, 8(49), 85492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Li Q, Li K, Chen L, Li W, Hou M, … Xu D (2013). Telomerase reverse transcriptase promotes epithelial–mesenchymal transition and stem cell-like traits in cancer cells. Oncogene., 32(36), 4203–4213. [DOI] [PubMed] [Google Scholar]
- 26.Tang B, Xie R, Qin Y, Xiao Y, Yong X, Zheng L, … Yang S (2015). Human telomerase reverse transcriptase (hTERT) promotes gastric cancer invasion through cooperating with c-Myc to upregulate heparanase expression. Oncotarget., 7(10), 11364–11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dikmen Z, Gellert G, Jackson S, Gryaznov S, Tressler R, Dogan P, … Shay J (2005). In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Research. 65(17), 7866–7873. [DOI] [PubMed] [Google Scholar]
- 28.Frink R, Peyton M, Schiller J, Gazdar A, Shay J, & Minna J (2016). Telomerase inhibitor imetelstat has preclinical activity across the spectrum of non-small cell lung cancer oncogenotypes in a telomere length dependent manner. Oncotarget., 7(22), 31639–31651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salloum R, Hummel T, Kumar S, Dorris K, Li S, Lin T, … Drissi R (2016). A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. Journal of Neuro-Oncology., 129(3), 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiappori AA, Kolevska T, Spigel DR, Hager S, Rarick M, Gadgeel S,….Schiller, JH. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann Oncol 2015;26(2):354–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascolo E, Wenz C, Lingner J, Hauel N, Priepke H, Kauffmann I, … Schnapp A (2002). Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. The Journal of Biological Chemistry., 277(18), 15566–15572. [DOI] [PubMed] [Google Scholar]
- 32.Damm K, Hemmann U, Garin-Chesa P, Hauel N, Kauffmann I, Priepke H, … Schnapp A (2001). A highly selective telomerase inhibitor limiting human cancer cell proliferation. The EMBO Journal., 20(24), 6958–6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryan C, Rice C, Hoffman H, Harkisheimer M, Sweeney M, & Skordalakes E (2015). Structural basis of telomerase inhibition by the highly specific BIBR1532. Structure with Folding & Design., 23(10), 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward R, & Autexier C (2005). Pharmacological telomerase inhibition can sensitize drug-resistant and drug-sensitive cells to chemotherapeutic treatment. Molecular Pharmacology., 68(3), 779–786. [DOI] [PubMed] [Google Scholar]
- 35.Ruden M, & Puri N (2013). Novel anticancer therapeutics targeting telomerase. Cancer Treatment Reviews., 39(5), 444–456. [DOI] [PubMed] [Google Scholar]
- 36.Dogan F, & Biray Avci C (2018). Correlation between telomerase and mTOR pathway in cancer stem cells. Gene., 641, 235–239. [DOI] [PubMed] [Google Scholar]
- 37.Doğan F, Özateş N, Bağca B, Abbaszadeh Z, Söğütlü F, Gasımlı R, … Biray Avcı Ç (2019). Investigation of the effect of telomerase inhibitor BIBR1532 on breast cancer and breast cancer stem cells. Journal of Cellular Biochemistry., 120(2), 1282–1293. [DOI] [PubMed] [Google Scholar]
- 38.Bashash D, Ghaffari S, Mirzaee R, Alimoghaddam K, Ghavamzadeh A (2013) Telomerase inhibition by non nucleosidic compound BIBR1532 causes rapid cell death in pre-B acute lymphoblastic leukemia cells Leukemia and Lymphoma 54(3), 561–568. [DOI] [PubMed] [Google Scholar]
- 39.Karran P, & Attard N (2008). Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nature Reviews., 8(1), 24–36. [DOI] [PubMed] [Google Scholar]
- 40.Reyes-Uribe P, Adrianzen-Ruesta M, Deng Z, Echevarria-Vargas I, Mender I, Saheb S, … Villanueva J (2018). Exploiting TERT dependency as a therapeutic strategy for NRAS-mutant melanoma. Oncogene., 37(30), 4058–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, … & DiCara D, (2012). A landscape of driver mutations in melanoma. Cell, 150(2), 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayward N, Wilmott J, Waddell N, Johansson P, Field M, Nones K, … Tembe V (2017). Whole-genome landscapes of major melanoma subtypes. Nature., 545(7653), 175–180. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G, Wu L, Mender I, Barzily-Rokni M, Hammond M, Ope O, … Somasundaram R (2018). Induction of telomere dysfunction prolongs disease control of therapy-Resistant Melanoma. Clinical Cancer Research., 24(19), 4771–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mender I, LaRanger R, Luitel K, Peyton M, Girard L, Lai T, … Shay J (2018). Telomerase-mediated strategy for overcoming non–small cell lung cancer targeted therapy and chemotherapy resistance. Neoplasia., 20(8), 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sengupta S, Sobo M, Lee K, Senthil Kumar S, White A, Mender I, … Drissi R (2018). Induced telomere damage to treat telomerase expressing therapy-resistant pediatric brain tumors. Molecular Cancer Therapeutics., 17(7), 1504–1514. [DOI] [PubMed] [Google Scholar]
- 46.Gazzaniga F, & Blackburn E (2014). An antiapoptotic role for telomerase RNA in human immune cells independent of telomere integrity or telomerase enzymatic activity. Blood., 124(25), 3675–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S, Rosenberg J, Donjacour A, Botchkina I, Hom Y, Cunha G, & Blackburn E (2004). Rapid inhibition of cancer cell growth induced by lentiviral delivery and expression of mutant-template telomerase RNA and anti-telomerase short-interfering RNA. Cancer research, 64(14), 4833–4840. [DOI] [PubMed] [Google Scholar]
- 48.Kedde M, le Sage C, Duursma A, Zlotorynski E, van Leeuwen B, Nijkamp W, … Agami R (2006). Telomerase-independent regulation of ATR by human telomerase RNA. The journal of biological chemistry., 281(52), 40503–40514. [DOI] [PubMed] [Google Scholar]
- 49.Baena-Del Valle J, Zheng Q, Esopi D, Rubenstein M, Hubbard G, Moncaliano M, … De Marzo A (2018). MYC drives overexpression of telomerase RNA (hTR/TERC) in prostate cancer. The journal of pathology: a journal of the pathological society of Great Britain and Ireland., 244(1), 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kheimar A, Trimpert J, Groenke N, & Kaufer BB (2018). Overexpression of cellular telomerase RNA enhances virus-induced cancer formation. Oncogene doi, 10.1038/s41388-018-544-1 [DOI] [PubMed] [Google Scholar]
- 51.Akincilar SC, Unal B, & Tergaonkar V (2016). Reactivation of telomerase in cancer. Cellular and Molecular Life Sciences, 73(8), 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cesare AJ, & Reddel RR (2010). Alternative lengthening of telomeres: models, mechanisms and implications. Nature reviews genetics, 11(5), 319. [DOI] [PubMed] [Google Scholar]
- 53.Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, … & Haber DA (2015). Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science, 347(6219), 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min J, Wright WE, & Shay JW (2017). Alternative lengthening of telomeres mediated by mitotic DNA synthesis engages break-induced replication processes. Molecular and cellular biology, Mol cell bio. 37(20) e-00226–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, … & Shih IM (2011). Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. The American journal of pathology, 179(4), 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henson JD, & Reddel RR (2010). Assaying and investigating alternative lengthening of telomeres activity in human cells and cancers. FEBS letters, 584(17), 3800–3811. [DOI] [PubMed] [Google Scholar]
- 57.Ramlee M, Wang J, Toh W, & Li S (2016). Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes, 7(8), 50 doi: 10.3390/genes7080050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, … & Muschel RJ,. (2012). Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell death & disease, 3(12), e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, … & Fernandez-Capetillo O (2011). A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nature structural & molecular biology, 18(6), 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, Van Der Horst CM, … & Peeper DS (2005). BRAF E600-associated senescence-like cell cycle arrest of human naevi. Nature, 436(7051), 720. [DOI] [PubMed] [Google Scholar]