Abstract
Metastasis is a complex systemic disease that develops as a result of interactions between tumor cells and their local and distant microenvironments. Local and systemic immune-related changes play especially critical roles in limiting or enabling the development of metastatic disease. Although anti-tumor immune responses likely eliminate most early primary and metastatic lesions, factors secreted by cancer or stromal cells in the primary tumor can mobilize and activate cells in distant organs in a way that promotes the outgrowth of disseminated cancer cells into macrometastatic lesions. Therefore, the prevention, detection, and effective treatment of metastatic disease require a deeper understanding of the systemic effects of primary tumors as well as predisposing hereditary and acquired host factors including chronic inflammatory conditions. The success of immunotherapy in a subset of cancer patients is an example of how modulating the microenvironment and tumor-immune cell interactions can be exploited for the effective eradiation of even advanced-stage tumors. Here, we highlight emerging insights and clinical implications of cancer as a systemic disease.
Keywords: metastasis, systemic effects, host factors
1. Introduction
Metastasis is the leading cause of cancer-associated mortality [1, 2]. Our ability to effectively treat metastatic disease has not changed significantly in the past few decades, emphasizing the importance of early detection and prevention of metastatic progression. Recent data from experimental models and clinical experience suggest that cancer is a systemic disease even at early stages, since primary tumors can alter their local and systemic environments in ways that affect metastasis development [3–5]. Disseminated and circulating tumor cells (DTCs and CTCs) can be detected in patients even with early stage disease [6] and although not all of these cells develop into fullblown metastatic lesions, their detection indicates metastatic spread and aids clinical prognostication [7]. Many tumor-derived factors systemically alter distant organs and prime resident mesenchymal and inflammatory cells to form both pre-metastatic and post-dissemination niches to foster metastatic outgrowth [8, 9]. But some primary tumors also secrete factors that inhibit metastases [10–13], suggesting a complex role for tumor-derived factors in metastasis development. Finally, tumor-educated stromal components, most notably bone marrow cells (BMCs), in turn, may act in a systemic manner by infiltrating primary and secondary sites where they exert pro- or anti-tumorigenic effects. For instance, in the process of systemic instigation BMCs undergo education, mobilization, and recruitment to tumors where they contribute to the development of a tumor microenvironment that fosters cancer progression and metastasis [3].
Indeed, early signs of metastatic disease include systemic changes in other organs such as splenomegaly (enlargement of spleen), thrombocytosis [14, 15] (increased platelet numbers), and neutrophilia [16] (increased neutrophil numbers), which have been shown to serve as independent prognostic factors for metastatic disease in various cancer types. Most of these comorbidities relate to inflammatory and immunological processes, and treatment of the underlying cause of the pathological complications may reduce tumor progression and metastasis. Supporting this idea, many systemic effects on host physiology have also been observed in animal models of cancer [14, 17] where their effect on metastatic progression has been tested experimentally. For instance, the use of an anti-platelet antibody to decrease platelet counts in ovarian carcinoma-bearing mice with thrombocytosis significantly reduced tumor growth and angiogenesis [14], while antibody-mediated depletion of Ly6G+ neutrophils decreased pulmonary metastasis in a mouse model of breast cancer [17]. Similarly, removal of the spleen, which is an important reservoir of myeloid cells such as neutrophils, reduced lung metastasis without affecting primary tumor growth [17].
Numerous systemic host factors related to chronic inflammation (e.g., obesity, asthma, psoriasis) also impact the risk of metastatic disease and patient outcomes. Additional risk factors associated with life-style, such as diet and exercise, also act on immune-related pathways [18], thus highlighting a key role of the immune system in determining metastatic outgrowth. In line with this, immunotherapy is one of the few curative therapies even for diffusely metastatic disease, although its success has been limited to certain cancer types and a subset of patients [19, 20]. Better understanding of tumor-induced and host-related systemic influences on metastasis should aid the discovery of new therapeutic approaches aimed at targeting disseminated disease. In this Review, we focus on the current understanding of drivers of metastasis, with particular focus on systemic pathophysiological processes that influence the biology of CTCs/DTCs and metastases. We discuss how these emerging paradigms of systemic interactions may guide therapeutic strategies designed to detect and prevent metastatic relapse.
2. Systemic spread of cancer cells: Disseminated and circulating tumor cells
During the complex, multi-step process of metastasis [21, 22], individual cancer cells and multi-cellular clusters detach from primary tumors and shed into the bloodstream and lymphatic system enabling systemic dissemination to distant sites and seeding of new metastatic colonies in secondary tissues [23] (Figure 1). Most CTCs quickly lose viability and do not survive due to detachment-induced anoikis, hemodynamic shear forces and mechanical trauma, or attack and clearance by the immune system, particularly by natural killer (NK) cells. Association with platelets or other CTCs to form multi-cellular clumps can enhance survival in circulation and protect tumor cells from immunosurveillance [7, 24]. Surviving CTCs and clusters arrest in capillaries of distant organs by entrapment or adhesion to the endothelium [25]. CTC clusters in particular are more likely to get physically lodged in the blood vessel lumen compared to single CTCs, although clusters are also capable of maneuvering through capillary-sized vessels as a single-cell chain [26]. Extravasated tumor cells penetrate the foreign tissue where they reside as DTCs (Figure 1). At this point, DTCs either stay dormant or colonize the tissue by expanding into clinically detectable macrometastases. Multi-cellular clusters also show advantages in survival and proliferation at secondary sites accumulating into increased metastatic efficiency [7], with the probability of a single CTC successfully forming a metastatic colony being utterly small.
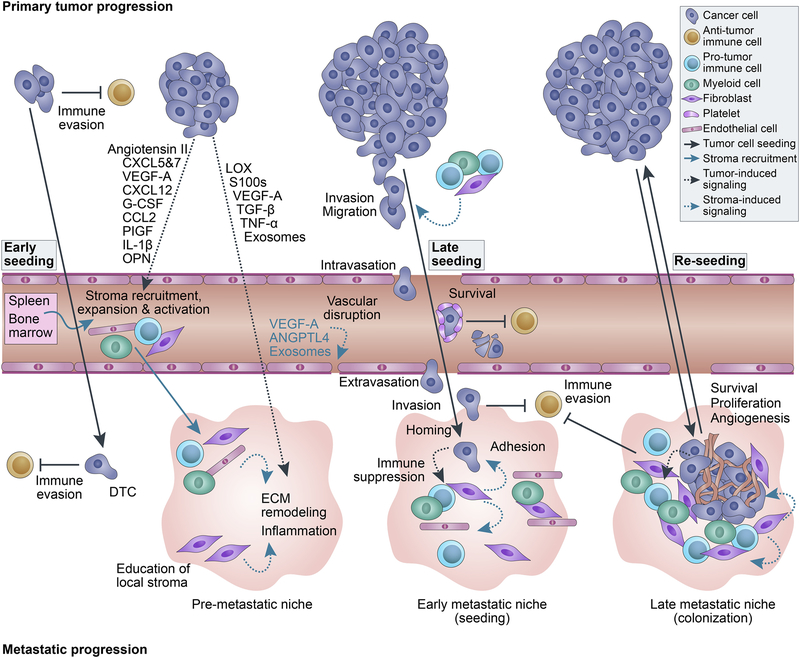
Figure 1. Mechanism of metastatic progression.
During primary tumor growth cancer cells can shed into the circulation and disseminate to distant sites, which may even occur at a very early stage of primary tumor development. Tumor-secreted factors can recruit, expand and activate various stromal cells, including spleen- and bone marrow-resident cells, and facilitate the formation of pre-metastatic niches at secondary sites. The recruited stromal cells aid the dissemination of carcinoma cells survival in circulation, and metastatic seeding and colonization. Immune evasion and angiogenesis are necessary steps of tumor progression mediated by tumor- and stroma-derived factors. Self-seeding of the tumor at later stages and re-seeding from metastatic lesions increase intratumoral heterogeneity and the risk of therapeutic resistance.
Recent technological advances have significantly improved our ability to detect, quantify, and molecularly characterize CTCs and DTCs. While DTCs are associated with poor clinical outcome, such as DTCs in bone marrow aspirates from breast cancer patients [6], analyses of CTCs in peripheral blood are more clinically appealing due to the relative ease with which they can be collected. Just like DTCs, CTCs are detectable in patients with most advanced stage cancers, including breast, prostate, and lung cancer, and they are associated with poor prognosis [7]. Moreover, changes in CTC numbers can reflect therapeutic response or resistance [27–29], and guide treatment decisions [30]. Both, DTCs and CTCs are detectable years after removal of the primary tumor, as was shown for CTCs in prostate cancer patients [31]. Because some CTCs may represent intermediaries between primary tumors and metastatic lesions, they have been characterized at the cellular and molecular level to better understand metastasis-initiating cells. A recent study in metastatic breast cancer patients demonstrated that a subpopulation of CTCs expressing EPCAM, CD44, CD47, and MET, harbored metastasis-initiating capabilities in immunocompromised mice, and higher numbers of these cells correlated with shorter overall survival and increased number of metastatic sites [32]. However, whether this specific subpopulation can initiate metastases in patients remains unclear. In addition to single-cell CTCs, CTC clusters can also be detected in the bloodstream of patients with various cancer types including lung [33] and breast carcinomas [34], although at a much lower frequency than single CTCs, and their presence is associated with adverse outcomes. Additionally, in primary tumors such clusters may be identifiable by their presence of leader cells that drive collective migration. Leader cells are histologically [35] and epigenetically [36] distinct in primary tumors, and may serve as useful predictive markers for risk of recurrence. Therapeutic targeting CTCs/DTCs may also be feasible and could benefit a subset of patients. For instance, in a small breast cancer patient cohort that received trastuzumab for treatment of HER2+ DTCs in the bone marrow, only patients whose marrow failed to clear (2 out of 10) eventually relapsed, while the other remained disease-free [37].
The ability to detect tumor cells outside of a visible tumor mass has led to attempts to correlate traditional histopathological markers with the presence of CTCs or DTCs; however, success in this area has been limited [4]. Many molecular markers, such as ER (estrogen receptor), PR (progesterone receptor), and HER2 in breast cancer, can be discordant between primary and metastatic tumors (and different metastatic sites), and are also heterogenous among CTCs [38] and DTCs [39]. This suggests that treatment designed based on primary tumor biopsies may not always be effective in metastatic disease. On the other hand, some metastatic tumors may respond to targeted therapies even if the target was not detected in the initial diagnostic biopsy. Our inability to accurately predict whether DTCs/CTCs will eventually form overt metastatic lesions hinders their clinical application. Hence, better characterization of DTCs/CTCs and delineation of molecular mechanisms that enable their outgrowth are needed prior to the design of therapeutic strategies to target them.
3. Evolutionary changes during systemic spread: The clonality of metastases
Recent single-cell genome sequencing and multi-color lineage tracing studies in both cancer patients [40, 41] and in animal models [7, 42–44] challenge the conventional model that each metastasis arises from a single primary tumor cell and instead reveal that most secondary lesions are composed of multiple genetically distinct subclones (Figure 2). However, whether a polyclonal seed develops into a polyclonal macrometastasis may depend on the organ site of metastasis. Data in a pancreatic cancer animal model demonstrated that while the majority of small metastatic lesions were polyclonal, metastases that grew out in the lung and liver drifted towards monoclonality, but peritoneal and diaphragmatic metastases remained polyclonal [42]. This suggests that some secondary sites may provide a more permissive state for the expansion of polyclonal seeds.
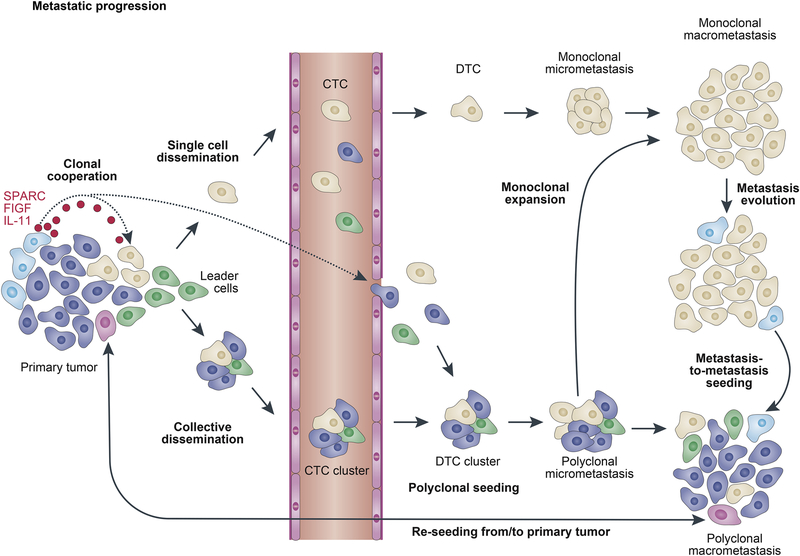
Figure 2. Clonality of métastasés.
Metastatic lesions can be monoclonal or polyclonal. Monoclonal seeding of circulating tumor cells (CTCs) shed from the primary tumor or monoclonal expansion of a subclone within a polyclonal metastasis can give rise to a monoclonal metastatic lesion. Polyclonal métastasés can emerge through multiple different mechanisms. Clonal cooperation promotes the metastatic abilities of poorly metastatic subclones. Collective migration and dissemination as CTC clusters lead to formation of polyclonal disseminated tumor cell (DTC) clusters at the secondary site. Re-seeding from the primary tumor or from other metastatic lesions can also contribute to metastatic heterogeneity.
Polyclonal metastases could arise by multiple different mechanisms including tumor-intrinsic and microenvironment-driven (Figure 2). Collective migration and spreading of cancer cells as a cohesive group [45] has been observed in preclinical [43] and clinical [46–48] studies, and could create polyclonal metastatic lesion. Studies using in vivo and in vitro models demonstrated that leader cells at invasive fronts displaying motile, mesenchymal features drive the invasion and dissemination events of multiple clones resulting in spread of polyclonal cell clusters that eventually metastasize together as CTC clusters [34, 36, 42, 43, 49]. Polyclonal metastases could also arise due to intratumor subclonal heterogeneity within primary tumors where certain subclones may directly or indirectly, through the local and systemic environment, enhance metastatic outgrowth. For example, in a xenograft model of breast cancer polyclonal tumors were more metastatic than their monoclonal counterparts and yielded polyclonal metastases, which was driven by clonal cooperation between FIGF- and IL11-expressing subclones [50]. Specifically, FIGF increases endothelial vessel leakiness, while IL-11 promotes angiogenesis and also leads to the recruitment and activation of neutrophils that form a premetastatic niche and enable the outgrowth of macrometastatic lesions composed of a mixture of cancer cells [51]. Similarly, one subclone could act in paracrine fashion to increase the metastatic potential of other subclones, as has been shown in pancreatic cancer wherein non-metastatic cancer cells secrete SPARC that increases the metastatic behavior of cancer stem cells [52]. Alternatively, polyclonal metastases could arise through re-seeding or metastasis-to-metastasis seeding as shown by deep-sequencing analyses in metastatic prostate cancer patients [40] and in animal models [53]. Interestingly, in prostate cancer patients, metastasis-to-metastasis spread occurred both as monoclonal and polyclonal seeding leading to increased intratumor genetic heterogeneity [40].
In patients with multiple distant metastatic lesions, metastases tend to be more closely related to each other than to the primary tumor, as observed in prostate cancer [40], medulloblastoma [54], and breast cancer [55, 56]. Furthermore, metastases located in the same organ are more closely related than those in different organs, as described in pancreatic and prostate cancer patients [40, 57]. Intriguingly, metastases within close physical proximity are also often more similar to each other than to more distant lesions [40], raising the question whether similarity between metastases in the same organ arises as a result of localized migration or from tissue-specific seeding. This also suggests that micro- and macroenvironments have distinct effects on the clonal evolution of cancer metastases by driving the selection for cancer cells with different properties and increasing cellular genetic heterogeneity within and between lesions. Whether subclonal competition for resources in the primary tumor could promote metastasis is an interesting, yet unresolved question. In support of the competition model, one recent study showed that multiple related tumor subclones in prostate cancer patients compete for dominance across the entirety of the host [40]. However, further mechanistic studies will be needed to dissect this competition process and its contribution to metastasis.
Besides the cellular and genetic composition of the tumor itself, germline genetic differences of the host can also impact both tumor development and metastasis. For example, host polymorphisms have been shown to influence metastasis susceptibility [58, 59]. A pioneering study that crossed male mice carrying the MMTV-PyMT oncogene to females of various inbred strains demonstrated that the maternal genotype significantly altered the metastatic capacity in the progeny and highlighted the significance of host genetic background for metastasis [59]. One such determinant of metastatic capability was later identified to be MMP9 as genetic ablation or pharmacologic inhibition in C57BL/6 mice drastically reduced lung metastasis burden without affecting primary mammary tumor growth [60]. In this model, MMP9 was predominately produced by neutrophils and related to angiogenesis in the lung metastases. Interestingly, the anti-metastatic outcome of MMP9 ablation was strain dependent, as FVB/N mice showed no effect on lung metastasis [60], suggesting that responses to MMP inhibition are controlled by genetic differences. Similarly, a study on hepatocellular carcinoma found while different strains of male mice developed liver cancer spontaneously or due to hepatocarcinogens, the primary differences between stains were in latency of the primary tumor and occurrence of pulmonary metastases [61]. Furthermore, different strains of inbred mice have a 10-fold range of response to growth factor-stimulated angiogenesis in the corneal micropocket assay [62], suggesting genetic factors could control angiogenic potential and thus progression of cancer. In line with this, single nucleotide polymorphisms (SNPs) in several genes, such as SIPA1, RRP1B, BRD4, and ARID4B have been reported to associate with risk of metastatic breast cancer [58]. Interestingly, a recent study identified LM01 as a neuroblastoma susceptibility gene that also functions as an oncogene and a metastasis-promoter in high-risk neuroblastoma through synergy with MYCN [63]. Specifically, transgenic expression of LM01 in a zebrafish model reduced latency and enhanced penetrance of neuroblastomagenesis through boosted proliferation while increased hematogenous dissemination and distant metastasis were linked to neuroblastoma cell invasion and migration and integrin expression.
4. Systemic cellular and molecular drivers of metastasis
While the intrinsic properties of cancer cells are important determinants of their metastatic ability and organ-site preference [22], non-neoplastic cells also have a major impact on metastatic spread and outgrowth. Tumor cells extensively communicate with cellular and non-cellular components of local and distant sites to facilitate the construction of metastasis-supportive environments even before tumor cell arrival at secondary organs by creating a pre-metastatic niche [5, 64] (Figure 1). Signaling between tumor and stromal compartments comprises a large set of factors including cytokines, chemokines, exosomes, circulating miRNAs, extracellular matrix (ECM) components, gap junctions, and mechanical cues [8, 64, 65]. Pre-metastatic niche formation in target organs is a complex process, involving systemic signaling, cell recruitment and activation, that collectively creates a more favorable microenvironment for DTCs. The tissue-specificity of pre-metastatic niches might be guided by exosomes secreted by organotropic cancer cells that preferentially fuse with resident cells at their predicted destination depending on exosomal integrin expression patterns [66]. For example, lung-tropic exosomes fuse with SPC+ epithelial cells and S100A4+ fibroblasts, while liver-tropic exosomes fuse with F4/80 Kupffer cells. This uptake prepares the premetastatic niche for cancer cell arrival by upregulation of different S100 proteins, such as S100P and S110A8 in the liver, and promotes pro-migratory and pro-inflammatory activities at the secondary sites (Figure 3). The pre-metastatic niche subsequently evolves into a metastatic niche upon direct interactions with metastasized cancer cells, and further recruitment of stromal cells and circulating factors. At this stage, DTCs need to evade and suppress cytotoxic leukocyte activity, such as CD8+ T cells and NK cells, and establish an immunosuppressive microenvironment.
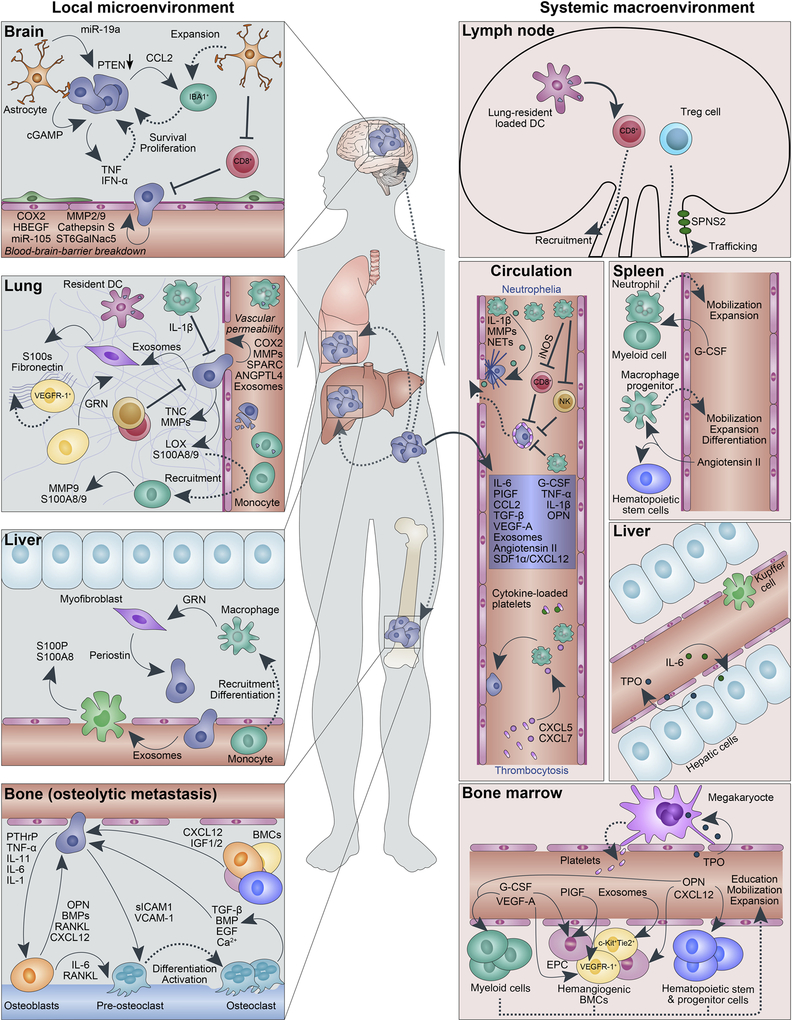
Figure 3. Local and systemic factors contributing to metastatic progression.
Primary tumors secrete various factors that act systemically to promote metastatic dissemination (blue box). These factors can affect stromal cells at distant sites (systemic macroenvironments), such as the spleen, liver and bone marrow, leading to their activation, expansion, and/or recruitment. Several cancer-related complications arise due to expansion of stromal cells, such as neutrophilia (increased neutrophil numbers) and thrombocytosis (increased platelet numbers). Activated and recruited stromal cells in turn can affect metastasis at the primary site (not shown), in circulation or at distant tissues (local microenvironments). Interactions of cancer cells and stromal cells at distant sites are tissue-specific as each secondary site varies in its physical properties and cellular and matrix composition. For instance, fenestrated endothelia in the bone and lung are likely permissive to tumor cell passage, while lung capillaries lined with a basement membrane and the blood-brain-barrier require specific mediators of cancer cell extravasation for successful metastatic seeding. Anti-tumor immune cells, such as cytotoxic CD8+ T cells which are expanded and recruited from the lymph node, can clear tumor cells from the circulation or at distant sites. Tumor cells gain immune protection through coating by platelets and actions of other immune and stromal cells, including neutrophils. The latter can also form neutrophil extracellular traps (NETs) to promote extravasation.
a. Secreted factors in metastatic progression
Tumor cell-derived cytokines and chemokines mobilize and recruit various local and distant stromal cells including leukocytes into primary and secondary sites (Figure 3). For example, Angiotensin II, CXCL12 (SDF-1α), G-CSF, and OPN mobilize bone marrow- and spleen-resident cells into circulation and promote tumor development and metastasis [3]. Similarly, in murine cancer models tumor-derived VEGF-A and PIGF trigger release of bone marrow-resident endothelial progenitors and VEGFR1+ BMCs that subsequently mediate tumor angiogenesis [67] and the formation of a premetastatic niche by increasing MMP9 expression in pre-metastatic lung endothelial cells via VEGFR-1/Flt-1 tyrosine kinase signaling [68] or upregulating fibronectin expression in lung fibroblasts that leads to the recruitment of pro-metastatic VEGFR1+ BMCs [69]. Tumor-secreted VEGF-A, TGF-β, and TNF-α also induce S100A8 and S100A9 expression in the lung that recruits circulating pro-metastatic CD11b+ myeloid cells in a VEGFR1-dependent manner [70]. Similarly, accumulation of tumor-secreted LOX recruited bone-marrow-derived myeloid cells to pre-metastatic niches in the lung [71]. Tumor-secreted factors also contribute to the evolution of paraneoplastic conditions. IL-6 released by ovarian carcinoma in a mouse model has been shown to increase hepatic TPO production, which in turn impinged on bone marrow megakaryocytes, resulting in an accelerated rate of platelet production [14]. Similar systemic effects have also been tied to factors released by tumor-associated stromal cells. CXCL12 is secreted by cancer-associated fibroblasts (CAFs) in primary breast tumors [72] and in a xenograft model of mammary carcinoma, endothelial progenitors and hematopoietic stem and progenitor cells were mobilized from the bone marrow in response to fibroblast-derived CXCL12 [73]. Importantly, elevated levels of many of these tumor- and stroma-derived cytokines are also detectable in primary tumors and plasma of patients with different cancer types and higher levels are associated with poor prognosis and increased metastatic burden [3]. Many of the mobilized cell populations are also expanded and rendered pro-tumorigenic by tumor-derived factors before their dissemination into circulation. For instance, melanoma exosomes educate BMCs toward a pro-metastatic phenotype through transfer of MET in a murine melanoma model [74] (Figure 3). Furthermore, these exosomes induce vascular permeability in mouse lungs, thereby further promoting metastatic progression of melanoma [74]. In fact, many tumor-induced systemically acting factors, such as COX2, ANGPTL4, and matrix metalloproteinases (MMPs), increase metastasis by stimulating vascular permeability, which is essential for extravasation to organs such as the lung or brain where the vasculature is lined with tightly adjoined endothelial cells [75]. Interestingly, a recent study of leptomeningeal metastasis, which occurs when cancer cells infiltrate the cerebrospinal fluid (CSF), found tumor-secreted Complement component 3 (C3) could bind and activate the C3a receptor on the epithelial cells of the choroid plexus thereby disrupting the blood-brain barrier [76]. This allowed plasma mitogens to enter the CSF and promote metastatic growth. Importantly, C3 expression in primary tumors from patients with different solid tumors correlated with clinical outcome and was predictive of leptomeningeal relapse, implying that blocking C3 signaling may be an effective therapy for the treatment of leptomeningeal metastasis.
b. The role of hematopoietic cells in metastatic progression
The key role of the immune system in the microenvironmental regulation of metastasis was highlighted in a recent genome-wide in vivo screen for regulators of metastatic colonization [77]. Using 810 mutant mouse strains, the authors identified 23 genes whose disruption modified lung metastatic abilities, noting that all but four of them associated with immune-related phenotypic traits. One of these factors, Spns2, regulates lymphocyte trafficking and its loss greatly reduces circulating lymphocyte numbers [77]. Nevertheless, mice lacking Spns2 were able to recruit higher percentages of effector T and NK cells at the secondary site, leading to increased tumor cell killing and overall decreased metastatic burden.
The systemic role of mobilized immune cell populations in metastasis is context-dependent, most notably for myeloid cell like neutrophils and monocytes. Gr1+CD11b+ myeloid cells, which encompass neutrophils and other granulocytes and strongly mediate tumor immunosuppression, secrete MMPs at the tumor-stroma interface of primary murine mammary carcinomas leading to improved tumor cell invasion and metastasis [78]. They can also be recruited to the lungs of mammary tumor-bearing mice where they alter the microenvironment and shift the balance of immune protection in favor of tumor promotion [79, 80]. Populations of Gr1+CD11b+ myeloid cells are significantly expanded in the bone marrow and spleens of tumor-bearing mice [81], in line with clinical observations of their elevated circulating numbers in cancer patients [82]. In murine models of lung metastasis, mammary tumors have been shown to elicit a systemic neutrophilic inflammatory cascade by secreting CCL2 and inducing IL-1β expression in tumor-associated macrophages, which led to systemic production of IL-17 by yδ T cells and G-CSF-dependent expansion and polarization of CD11 b+Ly6G+Ly6C+F4/80− neutrophils [83, 84]. The latter suppressed cytotoxic CD8+ T lymphocytes to promote cancer cell dissemination and metastatic outgrowth. Neutralizing CCL2 in the adjuvant setting, blocking IL-17 or G-CSF and absence of yδ T cells prevented neutrophil accumulation, downregulated the T-cell-suppressive phenotype and overall decreased pulmonary metastasis. These observations are in line with other studies in mammary tumor models in which recruitment of monocytes, such as macrophages and preosteoclasts, to the lung and bone by tumor-derived CCL2 ultimately led to increased metastasis at these secondary sites [85, 86]. However, if the abovementioned neutralization of CCL2 was administered not in the adjuvant, but in the neo-adjuvant context followed by cessation of the therapy, then inhibition of inflammation actually resulted in enhanced metastasis [84], in line with another report that adjuvant combination therapy with paclitaxel and an IL-1β inhibitor enhanced metastasis [87]. These studies highlight the complex, context-dependent role of immune cells in cancer progression in which timing of the treatment (e.g. neo-adjuvant vs. adjuvant) is a critical determinant of their effect on metastasis.
CD11 b+/Ly6G+ neutrophils mobilized by breast cancer-derived G-CSF have also been reported to inhibit NK cell function [17], thereby significantly increasing the intraluminal survival of CTCs (Figure 3). In these models, neutrophils also facilitated extravasation of CTCs by secreting IL-1β and MMPs. Likewise, in a murine model of metastatic mammary carcinoma, cancer cells induced the formation of neutrophil extracellular traps (NETs) at host sites which promoted metastasis of CTCs [88]. Such NETs were also detected in clinical samples from triple-negative breast cancer (TNBC) patients [88]. Importantly, as neutrophils have a very short half-life and their expansion is tumor-induced, surgical removal of the primary tumor led to their immediate reduction in animal models [89]. Similarly, neutrophil depletion significantly reduced pulmonary and lymph node metastasis in these models. In line with these pre-clinical observations, neutrophils have been suggested as markers of poor prognosis in a variety of cancers, including lung [90], pancreatic [91], ovarian [92], gastric [93], colorectal [94], and renal [95] cancer. Furthermore, elevated plasma G-CSF levels have been associated with severe leukocytosis (abnormally high leukocyte count) and poor prognosis [96, 97], while IL-1β secretion in primary breast cancers has been correlated with disease progression [98]. Finally, an “IL-1 signature” in blood leukocytes from patients with metastatic HER2-negative breast cancer was linked with poor prognosis [98], but could be attenuated by IL-1 blockade, suggesting that patients with IL-1 signature genes could benefit from therapy targeting IL-1 signaling.
Conversely, studies using pre-clinical models have also shown an anti-metastatic effect for neutrophils by promoting tumor cell killing and preventing metastatic establishment [99]. In agreement, a recent report demonstrated that early-stage primary breast carcinomas induce systemic inflammation to mobilize IL-1β-expressing neutrophils that infiltrate distant metastases and prevent their subsequent colonization by maintaining them in a dormant state, but importantly, do not result in the clearance of metastatic cells [13]. To this end, analysis of tumor specimens of breast cancer patients with lymph node-positive disease who are at increased risk of distant metastasis, revealed that high IL-1β levels in primary tumors associated with improved overall and distant metastasis-free survival [13]. At first glance, these various reports may seem discrepant; however, neutrophils and monocytes are very heterogeneous and plastic cell populations with varying phenotypes under different conditions. A striking confirmation of the importance of context and neutrophil heterogeneity was described in a recent paper discussed above focusing on mechanisms by which clonal cooperation in primary tumors promotes metastasis in an experimental model of breast cancer [51]. In this study the depletion of neutrophils could either promote or decrease lung metastases depending on the expression of IL11 and FIGF in the primary tumor. Singlecell RNA-sequencing revealed that lung neutrophils had activated pro-metastatic profiles when primary tumors expressed doxycycline-inducible IL11 and FIGF, but more naive, presumably anti-tumor, profiles in mice not fed with doxycycline whose primary tumors lacked IL11 and FIGF expression. These data emphasize the functional and clinical relevance of cellular heterogeneity of both cancer and stromal cells within tumors and at distant sites.
Aside from cellular heterogeneity, spatial heterogeneity also contributes to differential tumor progression and clinical outcomes. Recent studies on TNBC found spatially distinct tumor immune microenvironments can stratify TNBC patients [100, 101]. While cold TNBCs have only very few immune cells, most of which are macrophages, some TNBCs show immune cell accumulation either within the tumor (mixed) or specially segregated from the tumor (compartmentalized) [100]. Importantly, patients with a mixed tumors have better outcomes than those harboring compartmentalized tumors. In line with this, another study on TNBC showed that an immunoreactive microenvironment with tumoral infiltration of granzymeB+CD8+ T cells, a type 1 IFN signature, and elevated expression of immune inhibitory molecules including IDO and PD-L1 is linked to good outcomes, while a distinct poor-outcome immunomodulatory microenvironment exhibits stromal restriction of CD8+ T cells, stromal expression of PD-L1, and enrichment for signatures of cholesterol biosynthesis [101].
Interestingly, a recent study using intravital two-photon imaging directly observed the arrival of CTCs and subsequent host interactions in the mouse lung thereby shedding light on the early roles of different immune cell populations at the secondary site. Shear flow in the capillaries generated immune-reacting microparticles through fragmentation of CTCs [102]. These particles were taken up by waves of distinct myeloid subpopulations: first, by neutrophils, then conventional monocytes, followed by non-alveolar macrophages, patrolling monocytes and dendritic cells (DCs). While monocytes and macrophages were able to extravasate into the lung parenchyma after microparticle ingestion, where they dominated early metastatic lesions and promoted their outgrowth, lung-resident DCs migrated to the lymph nodes to activate an antimetastatic T cell response [102].
Thrombocytosis is frequently observed in cancer patients and correlates with worse prognosis [103]. In line with this, platelets facilitated metastasis of murine colon cancer cells by secreting CXCL5 and CXCL7, which recruit CXCR2+ granulocytes to sites of CTCs trapped inside the lumen [104] (Figure 3). Preventing host-tumor cell interactions by interfering with platelet function or granulocyte recruitment impaired tumor cell seeding and metastasis. Similarly, tumor-derived cytokines can be absorbed by platelets, which are recruited to responding murine tumor sites where they aid vessel formation and tumor progression, which can be inhibited by aspirin administration [105].
c. The role of stromal cells in metastatic progression
Other non-inflammatory resident and distant stromal cells can also be educated and recruited to primary and secondary tumors where they collectively shape metastatic progression. For example, endothelial progenitor cells can be recruited to tumors to promote tumor angiogenesis [67]. OPN secreted by TNBC activates and mobilizes BMCs not only to primary tumors of tumor-bearing mice, but also to weakly metastatic DTCs in the lungs, where they secrete GRN, causing resident fibroblasts to adopt a CAF phenotype and express pro-inflammatory and matrix-remodeling genes that further support tumor progression [106, 107] (Figure 3). Similarly, in models of pancreatic cancer, macrophage-derived granulin promoted metastasis by stimulating periostin secretion by myofibroblasts and liver fibrosis [108]. Interestingly, recent single-cell sequencing approaches have identified spatially and functionally distinct subclasses within stromal populations, such as CAFs [109, 110]. Three functionally distinct CAF subclasses identified in a genetically engineered mouse model of breast cancer were attributable to different origins, including the perivascular niche, the mammary fat pad and the transformed epithelium [109]. Gene profiles for each CAF subtype also held independent prognostic capability in clinical cohorts by association to metastatic disease. Another study found four distinct CAF subsets (CAF-S1 to CAF-S4) in human breast cancer with distinct properties and levels of activation [110]. These subclasses accumulated differentially in juxta-tumor compared to tumors and among different breast cancer subtypes. Interestingly, TNBC could be divided into two subgroups according to enrichment of either CAF-S1 or CAF-S4. Unlike CAF-S4-, CAF-S1-enriched TNBC exhibited an immunosuppressive environment with high content in FOXP3+T cells and low infiltration of CD8+ T cells. Specifically, by secreting CXCL12, CAF-S1 attracted CD4+CD25+ T cells, retained them through OX40L, PD-L2, and JAM2, promoted their survival and differentiation into CD25Hl9hFOXP3Hl0h T cells through B7H3, CD73, and DPP4. Finally, in contrast to CAF-S4, CAF-S1 enhanced the regulatory T cell capacity to inhibit T effector proliferation.
The resident stromal cells at secondary tissue sites can also affect metastatic outgrowth, either by suppressing or enhancing survival of DTCs and their progression into macrometastatic lesions. Preclinical studies have analyzed the stromal contribution to metastasis at various secondary sites, including the brain. For example, astrocyte-induced downregulation of PTEN by exosomal miR-19a led to increased CCL2 secretion by brain-metastatic tumor cells which in turn recruited brain-derived IBA1+ myeloid cells [111]. The latter reciprocally enhanced the outgrowth of brain metastatic tumor cells. Importantly, PTEN loss in brain metastases corresponded to higher CCL2 expression and IBA1+ myeloid cell recruitment in cancer patients, validating the clinical significance of these findings. Similarly, brain-metastatic murine mammary and lung cancer cells can assemble gap junctions with astrocytes to transfer cGAMP, which triggered the production of inflammatory cytokines IFN-α and TNF through the STING pathway [112]. These factors in turn stimulated the STAT1 and NF-kB pathways in the brain metastatic cells, thereby supporting tumor growth and chemoresistance. Using another murine brain metastasis model, a recent study demonstrated that a subpopulation of reactive astrocytes surrounding metastatic lesions expressed phospho-STAT3 (pSTAT3), which modulated innate and adaptive immune responses that cumulatively promoted metastatic outgrowth [113]. Specifically, pSTAT3+ astrocytes inhibited activation of CD8+ T cells, while promoting expansion of the CD74+IBA1 + microglia and macrophage populations. Given that almost 90% of all brain metastases are pSTAT3+ and that they correlate with worse clinical outcome [113], this immune response may also be important in promoting brain metastasis in cancer patients. In contrast to this cooperative tumor-stroma interactions at the metastatic site, a recent study found DTCs from lung and mammary carcinoma models use L1CAM to spread on capillaries in the perivascular niche of multiple secondary organs, thereby displacing resident pericytes [114], which use L1CAM for perivascular spreading under normal conditions. L1CAM activates mechanotransduction effectors YAP and MRTF in DTCs and enables their outgrowth upon their infiltration of target organs and after they exit from a period of dormancy. Overall, these findings signify the dynamic and reciprocal cross-talk between tumor cells and the metastatic niche in driving tumor.
6. Systemic host factors affect metastatic outcomes
Underlying host physiological processes have also been implicated in metastatic and in clinical outcome. For instance, age-related changes may influence disease progression, because aging has multiple effects on tissue homeostasis with either pro- or anti-tumorigenic consequences. For example, age-associated senescent osteoblasts have been shown to establish a bone metastatic niche by secreting IL-6 to support breast cancer metastases [115] and inhibitors of the p38MAPK-MK2 pathway limited metastatic outgrowth in this model [116]. Similarly, aged fibroblasts secrete a Wnt antagonist, sFRP2, which after activating a multi-step signaling cascade in melanoma cells ultimately results in loss of a key redox effector, APE1 [117]. This loss renders melanoma cells more sensitive to oxidative stress, increases their resistance to targeted therapy, and augments angiogenesis and metastasis. Hematopoietic age was also shown to be an important determinant of TNBC progression [118]. Consistent with clinical observations that younger TNBC patients have worse prognosis compared to older women [119], a recent study found that TNBC is more aggressive in young compared to old mice due to the increased responsiveness of Sca1+cKit− BMCs to TNBC-derived signals including OPN [118]. In this study, young BMCs rescued tumor growth in old mice. On the other hand, age is associated with increasing immune dysfunction and the implications of these age-related changes to cancer pathogenesis and progression have yet to be understood.
Epidemiological evidence indicates that obesity is associated with higher cancer risk [120] and with higher incidence of metastasis [121, 122]. In line with this, a recent study of mouse mammary tumor models confirmed that obesity-associated inflammation promotes metastatic progression by increasing lung neutrophilia, which is further intensified by the primary tumor [123]. The increase in lung neutrophils translated to increased lung metastasis in a GM-CSF- and IL-5-dependent fashion. Importantly, weight loss was sufficient to reverse these effects by reducing circulating GM-CSF and IL-5 levels in both mouse models and humans [123]. In addition, obesity impaired mouse and human NK cell anti-tumor activity by perturbing their cellular metabolism and trafficking [124]. On the other hand, recent data has demonstrated that obesity is associated with better outcomes upon immune and targeted therapies [125]. In this retrospective, multi-cohort analysis metastatic melanoma patients that responded to such therapies disproportionately included overweight patients. In line with these clinical observations, a study across multiple species and tumor models demonstrated that while obesity resulted in increased immune aging, tumor progression and leptin-induced, PD-1-mediated T cell dysfunction, it was also associated with increased efficacy of PD-1/PD-L1 blockade in both tumor-bearing mice and in cancer patients [126].
Other life-style and ethnicity-related differences may contribute to metastasis development, clinical outcomes, and therapy resistance. For example, diet can influence tumor and metastatic progression and treatment sensitivity [18, 127]. In lymphoma, melanoma, and colorectal cancer models, a moderate reduction in protein, but not carbohydrate intake, without overall calorie changes, induced the unfolded protein response (UPR) in carcinoma cells through activation of the IRE1α/RIG1 pathway, which in turn induced cytokine production and a tumor-specific cytotoxic T cell response [127]. Likewise, epidemiological evidence indicates that regular exercise could decrease cancer incidence and improve clinical outcomes [18, 128–130]. Exercise may regulate tumor growth through physical (e.g., increased blood flow and shear stress on the vascular bed) and endocrine (e.g., stress hormones and myokines) mechanisms, thereby increasing the mobilization and infiltration of innate and cytotoxic immune cells into the tumor microenvironment [131, 132]. For example, a recent study using five different mouse cancer models showed that voluntary running led to increased accumulation and activation of NK cells in an epinephrine- and IL-6-dependent manner, thereby reducing tumor growth by 60% [131]. Similarly, daily stretching for 10 minutes reduced tumor growth by more than 50% in a mouse orthotopic breast cancer model through activation of cytotoxic immune responses [132].
Pre-clinical evidence also exists in support of the idea that systemic changes associated with pregnancy can affect outgrowth of incipient breast tumors. To this end, mouse models using human breast cancer xenografts show that systemic estrogen signaling helped mobilize and recruit pro-angiogenic myeloid cells from the bone marrow to distant tumor sites, promoting tumor growth of even ER-negative breast carcinoma [133, 134]. Similarly, after pregnancy involution-associated inflammation in rodent models is defined by a dramatically increased immune cell infiltrate including immunosuppressive myeloid and FoxP3+ regulatory T cells [135], and an impairment of antigen-specific activation of T cells [136]. This involution-associated immune suppression may contribute to the tumor growth-promoting attributes of the involuting mammary gland and may also negatively impact the outcome of patients diagnosed with breast cancer within five years postpartum [137]. Importantly, ibuprofen treatment during involution reduced tumor growth and enhanced anti-tumor immunity in animal models [138], implying that ibuprofen use in the postpartum period may be beneficial to reduce breast cancer risk and improve outcomes.
Chronic inflammation may represent another risk factor for the development of metastases, as has been suggested for asthma in lung metastasis [139]. Allergy-induced pulmonary inflammation in a syngeneic melanoma mouse model caused the lungs to serve as a target tissue for metastasis through recruitment of CTCs in a CD4+ T cell-dependent manner, which was attenuated by asthma treatment [139]. Interestingly, the incidence of asthma was higher among breast cancer patients with lung metastases than in those without [90], suggesting that ameliorating asthma-associated pulmonary inflammation may benefit breast cancer patients with this disease. Similarly, in a mouse model of arthritis, systemic inflammation significantly increased breast cancer metastases to lungs and bones [140]. The pre-metastatic lungs of arthritic mice were highly inflamed and treatment with an anti-IL-17 antibody significantly reduced inflammation and metastatic burden [140]. In psoriasis, lymphocyte-derived IL-17 regulates neutrophil expansion via systemic induction of G-CSF [141] - a mechanism by which yδ T cells have been shown to increase pulmonary metastasis in mouse models of breast cancer [89].
Surgical resection of primary tumors may be an additional risk factor for metastatic relapse, particularly in breast cancer patients some of whom develop distant recurrence soon after primary tumor resection. A recent study in a mouse model of dormancy showed a systemic inflammatory response initiated as part of a wound-healing response to a primary tumor resection surgery promoted the emergence of metastatic tumors that were previously restricted by a tumor-specific T cell response [142]. Furthermore, perioperative anti-inflammatory treatment markedly reduced tumor outgrowth in this model. However, in another model of metastatic mammary carcinoma surgical resection of the primary tumor did not affect metastatic outgrowth [51], suggesting that surgery could have variable effects on metastatic outcome that may be tumor- and/or host-specific. Hence, it will be necessary to delineate the effect of surgery on metastatic outgrowth in order to assess which patients would benefit from a perioperative anti-inflammatory treatment to reduce the risk of early metastatic recurrence.
7. Metastatic latency
In certain cancer types, such as prostate cancer and ER+ breast cancer, metastasis can develop years or even decades after the removal of a primary tumor [22]. The long latency period between initial treatment and eventual metastatic recurrence suggests that some DTCs may persist in a dormant state for a long time. Systemic processes such as an anti-tumor immune response may play a role in both the dormancy and “awakening” of these cells. Clinical observations describing that immunosuppressed organ transplant recipients develop metastasis from donors who have been cured of cancer years (or even decades) earlier [75] support this hypothesis. Findings from human lung and breast cancer xenograft models suggest that latent DTCs may reside in a stem cell-like state regulated by the SOX family of transcription factors and actively silence WNT signaling to enter quiescence and evade innate immunity, particularly NK cell-mediated clearance [143]. The stem cell-like characteristics, marked by expression of SOX2 and SOX9, also potentiate metastatic outgrowth under permissive conditions. In a recent study, using a genome-wide CRISPR screen MSK1 was identified as a key regulator of metastasis dormancy in ER+ breast cancer by modulating GATA3 and FOXA1 expression and luminal differentiation [144]. Loss of MSK1 increased breast cancer cell bone homing and growth capacities. In line with this, in ER+ breast cancer patients, early metastasis was associated with low tumor MSK1 expression suggesting that MSK1 could be used for the stratification of ER+ breast cancer patients into low and high risk for recurrence groups. Similarly, comprehensive molecular profiling of breast tumors in combination with mathematical modeling was able to identify ER+ breast cancer patients who are at risk for late metastatic recurrence [145]. These results suggest that in at least a subset of cases metastatic dormancy may be determined by tumor cell intrinsic factors. However, besides tumor-specific factors, growth inhibitory signals from the host microenvironment and the perivascular niche, including TGF-β and BMP, can also contribute to metastatic dormancy [146]. The ultimate determinants of whether or not a given tumor cell or population of cells can give rise to a clinically significant metastasis may thus include not only the biology of the cancer cells themselves but also the physiologic state of the host.
8. Preventing and treating metastatic disease
The design of effective clinical interventions requires a deeper understanding of “seed” and “soil” that allow metastatic outgrowth. While there has been substantial effort to uncover the molecular determinants of cancer progression and organotropism, the clinical translation of these findings has been challenging. Reliable biomarkers that can be used to accurately identify either patients at risk of distant metastases or the organ specificity of metastases are still lacking. Since metastasis in many patients is a recurrence after surgical removal of the primary tumor and systemic therapy, drivers of metastatic outgrowth and therapeutic resistance cannot be distinguished. Moreover, targeted therapies directed at tumor-stromal interactions, while promising, are still in early stages of development, with the exception of immune checkpoint blockade therapy in some tumor types. Furthermore, how systemic responses to malignancy could be used to design effective therapies is still an area of active research. Nevertheless, consideration of cancer as a systemic disease opens a number of potential preventative and therapeutic avenues and integrates the patient’s general health and life-style in cancer treatment.
Circulating factors derived from cancer cells or tumor-associated stromal cells that induce systemic host responses could be used as biomarkers to detect or predict risk of recurrence [8, 65, 147]. Some circulating tumor markers are already used in the clinic, such as PSA for prostate cancer, CA-125 for ovarian cancer, AFP for liver cancer, and HCG for germ cell tumors, including testicular and ovarian cancers [148]. In addition, circulating cell-free nucleic acids such as circulating tumor DNA (ctDNA) and miRNAs are also promising biomarkers. ctDNAs are detected and primarily tracked based on tumor-specific genetic and epigenetic alterations. For instance, whole-genome sequencing of ctDNA was used to identify cancer-associated chromosomal markers in a subset of women with breast cancer [149]. Similarly, cancer-derived exosomes and their tumor-specific cargo have been proposed as promising diagnostic and prognostic biomarkers [66, 74, 150]. Finally, improvements in imaging technology have opened avenues for early detection of some cancer types, and some of these also allow for the earlier detection of metastatic spread. To this end, systemic tumor-associated factors could be incorporated into clinical practice as non-invasive imaging biomarkers.
The ability to identify tumors capable of eliciting a host systemic response or responding to changes in the host systemic environment will carry significant implications for future risk stratification strategies. For example, the identification of changes in the systemic environment may allow for more accurate classification of patients based on likelihood of relapse. Ultimately, this knowledge may enable oncologists to more accurately identify patients who may benefit from specific adjuvant therapies at a time when the progression to overt metastatic disease can still be prevented or at least significantly delayed. In addition, circulating stromal cells, particularly immune cells, may aid the prognostic assessments of malignancies. For example, the ratio of circulating neutrophils to lymphocytes has been reported to associate with outcome for patients with various cancer types, particularly advanced colon and pancreatic cancers, and could be used to predict survival and stratify treatment [91, 92, 94]. Circulating C-reactive protein and albumin measuring systemic inflammation have been used as independent prognostic factor for cancer patients in a variety of clinical scenarios [151].
One can envision opportunities for therapeutic intervention at multiple steps along the metastatic cascade. For example, targeting cancer-derived systemic factors, such as cytokines, that trigger host responses to ultimately support disease progression may serve as a means to interdict the systemic lines of metastatic support. Circulating factors could relatively easily be inhibited or restored, depending on their function in metastatic progression, as well as measured non-invasively (e.g., a blood test), enabling the efficacy of their therapeutic targeting to be monitored and adjusted during therapy. For example, in a preclinical model of non-small-cell lung cancer anti-OPN antibodies were shown to inhibit the growth of metastatic lesions in the lungs [152]. Similarly, inhibition of GRN prevented hepatocellular carcinoma metastasis in a preclinical mouse model [153].
Pre-clinical efforts to target the metastatic microenvironment at early stages of dissemination have also proven effective. Mammary tumor models demonstrated that bisphosphonates generate metastasis-suppressive hematopoietic myeloid/osteoclast progenitor cells [154, 155]. However, G-CSF promoted resistance to the bisphosphonate zoledronic acid (ZA), and breast cancer patients with high circulating G-CSF experienced significantly worse outcomes with adjuvant bisphosphonates [155].
As primary tumors can mobilize pro-tumorigenic BMCs to aid the outgrowth of disseminated tumors, specific therapies targeting these long-range systemic lines of molecular communication may have clinical significance. In a recent preclinical study of systemic instigation by luminal breast carcinomas, aspirin treatment abrogated the pro-angiogenic role of mobilized platelets [105], in accordance with clinical data describing that aspirin use resulted in a modest decrease in cancer risk and progression of breast [156] and colorectal [157] cancer, particularly in patients with PIK3CA mutant colorectal tumors [158]. Furthermore, a recent breast cancer study demonstrated that the prometastatic platelet function can be directly inhibited by tamoxifen [159]. Some evidence suggests that systemic tumor-induced effects should be targeted early during the metastatic cascade. For instance, depletion of neutrophils in a mouse model of breast cancer was more effective when applied during primary tumor growth than after the removal of the primary tumor [89]. However, the generalizability of this finding is still not clear.
In the past few years much effort has gone into the application of immunotherapy with promising results in a subset of patients [19]. The identification of new methods to reactivate cytotoxic immune function or induce immunosurveillance has become crucial for the development of effective therapeutic strategies. While some preclinical studies demonstrated that depletion of specific immune cell types (e.g., neutrophils or yδ T cells) decreased metastatic incidence [17, 89], others have reported increased metastatic colonization when neutrophil-derived cytokines were depleted at a particular time in early dissemination [13, 84, 160]. However, it is unlikely that such approaches would be feasible in cancer patients. Indeed, clinical success will surely rely on understanding the role of systemically acting factors at all stages of disease progression. Here, more tumor-specific targeting approaches will be required to avoid general immune-related complications, as those seen even with immune checkpoint inhibitors that have already been approved for clinical use [19]. Studies will also need to address when such immunotherapies will be most effective during the metastatic cascade, especially with cell populations whose effects may be context-dependent.
9. Summary and future directions
Considering cancer as a systemic disease opens avenues for novel ways to target metastasis, which is responsible for most cancer-related deaths. Future targeting of the metastatic process is likely to involve three critical steps. First, it will be important to identify and characterize local and systemic factors that facilitate the establishment of a metastatic tumor and develop ways to interfere with these. Second, it will be equally important to develop efficient and reproducible ways to screen individual patients for the risk of developing metastatic disease based on both host- and tumor-specific factors. This can be accomplished by performing detailed molecular profiling of both cancer and host cells at primary and metastatic sites as well as assessing the immune status of the host (e.g., past infectious diseases, microbiome). Third, it would also be important to consider the effects of cancer treatment on the risk of metastatic recurrence as ineffective treatment not only fails to limit tumor growth, but may inadvertently accelerate disease progression. Mathematical modeling of tumor evolution considering all known host and tumor factors as well as treatment design will likely aid the design of individualized cancer therapies. Because some of the tumor-promoting systemic effects are linked to modifiable factors such as chronic inflammation, obesity, and exercise, many cancers and metastatic relapses are potentially preventable. Basic pre-clinical and clinical studies have led to a large knowledge base regarding the underlying biology and clinical presentation of metastatic tumors. The next steps will involve integrating information about the systemic nature of cancer signaling into ongoing development of individualized therapy.
ACKNOWLEDGEMENTS
We would like to thank members of the Polyak lab for their critical reading of this manuscript. This work was supported by grants from the Breast Cancer Research Foundation and the National Cancer Institute (CA197623). We apologize to all authors whose publication we may have omitted due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS
K. Polyak serves on the scientific advisory boards of Mitra Biotech and Acrivon THerapeutics.
REFERENCES
- [1].Chaffer CL, Weinberg RA, A Perspective on Cancer Cell Metastasis, Science 331(6024) (2011) 1559–1564. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA: A Cancer Journal for Clinicians 68(1) (2018) 7–30. [DOI] [PubMed] [Google Scholar]
- [3].McAllister SS, Weinberg RA, The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis, Nat Cell Biol 16(8) (2014) 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Redig AJ, McAllister SS, Breast cancer as a systemic disease: a view of metastasis, Journal of internal medicine 274(2) (2013) 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Celiá-Terrassa T, Kang Y, Metastatic niche functions and therapeutic opportunities, Nature Cell Biology 20(8) (2018) 868–877. [DOI] [PubMed] [Google Scholar]
- [6].Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K, A pooled analysis of bone marrow micro meta stasis in breast cancer, N Engl J Med 353(8) (2005) 793–802. [DOI] [PubMed] [Google Scholar]
- [7].Aceto N, Toner M, Maheswaran S, Haber DA, En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition, Trends Cancer 1(1) (2015)44–52. [DOI] [PubMed] [Google Scholar]
- [8].Quail DF, Joyce JA, Microenvironmental regulation of tumor progression and metastasis, Nature medicine 19(11) (2013) 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, Rickelt S, Severe N, Baryawno N, Faget J, Savova V, Zemmour D, Kline J, Siwicki M, Garris C, Pucci F, Liao HW, Lin YJ, Newton A, Yaghi OK, Iwamoto Y, Tricot B, Wojtkiewicz GR, Nahrendorf M, Cortez-Retamozo V, Meylan E, Hynes RO, Demay M, Klein A, Bredella MA, Scadden DT, Weissleder R, Pittet MJ, Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils, Science 358(6367) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J, Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma, Cell 79(2) (1994) 315–28. [DOI] [PubMed] [Google Scholar]
- [11].Kang SY, Halvorsen OJ, Gravdal K, Bhattacharya N, Lee JM, Liu NW, Johnston BT, Johnston AB, Haukaas SA, Aamodt K, Yoo S, Akslen LA, Watnick RS, Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1, Proc Natl Acad Sci U S A 106(29) (2009) 12115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kubo H, Mensurado S, Gonçalves-Sousa N, Serre K, Silva-Santos B, Primary Tumors Limit Metastasis Formation through Induction of IL15-Mediated Cross-Talk between Patrolling Monocytes and NK Cells, Cancer Immunology Research 5(9) (2017) 812–820. [DOI] [PubMed] [Google Scholar]
- [13].Castaño Z, San Juan BP, Spiegel A, Pant A, DeCristo MJ, Laszewski T, Ubellacker JM, Janssen SR, Dongre A, Reinhardt F, Henderson A, Del Rio AG, Gifford AM, Herbert ZT, Hutchinson JN, Weinberg RA, Chaffer CL, McAllister SS, IL-1beta inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization, Nat Cell Biol 20(9) (2018) 1084–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stone RL, Afshar-Kharghan V, Causes and Consequences of Cancer-Associated Thrombocytosis, Blood 122(21) (2013) SCI-33–SCI-33. [Google Scholar]
- [15].Stravodimou A, Voutsadakis IA, Pretreatment Thrombocytosis as a Prognostic Factor in Metastatic Breast Cancer, International Journal of Breast Cancer 2013 (2013) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E, Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis, J Natl Cancer Inst 106(6) (2014) dju124. [DOI] [PubMed] [Google Scholar]
- [17].Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, lannello A, Iwamoto Y, Cortez-Retamozo V, Kamm RD, Pittet MJ, Raulet DH, Weinberg RA, Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells, Cancer Discov 6(6) (2016) 630–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kerr J, Anderson C, Lippman SM, Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence, The Lancet Oncology 18(8) (2017) e457–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wei SC, Duffy CR, Allison JP, Fundamental Mechanisms of Immune Checkpoint Blockade Therapy, Cancer Discovery 8(9) (2018) 1069–1086. [DOI] [PubMed] [Google Scholar]
- [20].Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im S-A, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA, Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer, New England Journal of Medicine 379(22) (2018) 2108–2121. [DOI] [PubMed] [Google Scholar]
- [21].Gupta GP, Massague J, Cancer metastasis: building a framework, Cell 127(4) (2006) 679–95. [DOI] [PubMed] [Google Scholar]
- [22].Lambert AW, Pattabiraman DR, Weinberg RA, Emerging Biological Principles of Metastasis, Cell 168(4) (2017) 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kang Y, Pantel K, Tumor cell dissemination: emerging biological insights from animal models and cancer patients, Cancer Cell 23(5) (2013) 573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lou XL, Sun J, Gong SQ, Yu XF, Gong R, Deng H, Interaction between circulating cancer cells and platelets: clinical implication, Chinese journal of cancer research = Chung-kuo yen cheng yen chiu 27(5) (2015) 450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen E.l., Lyden D, Bissell MJ, The perivascular niche regulates breast tumour dormancy, Nat Cell Biol 15(7) (2013) 807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Au SH, Storey BD, Moore JC, Tang Q, Chen YL, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O’Keefe R, Haber DA, Maheswaran S, Langenau DM, Stott SL, Toner M, Clusters of circulating tumor cells traverse capillary-sized vessels, Proc Natl Acad Sci U S A 113(18) (2016) 4947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW, Hayes DF, Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer, Clin Cancer Res 12(21) (2006) 6403–9. [DOI] [PubMed] [Google Scholar]
- [28].Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW, Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival, Clin Cancer Res 12(14 Pt 1) (2006) 4218–24. [DOI] [PubMed] [Google Scholar]
- [29].Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S, Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition, Science 339(6119) (2013) 580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Helissey C, Berger F, Cottu P, Dieras V, Mignot L, Servois V, Bouleuc C, Asselain B, Pelissier S, Vaucher I, Pierga JY, Bidard FC, Circulating tumor cell thresholds and survival scores in advanced metastatic breast cancer: the observational step of the CirCe01 phase III trial, Cancer Lett 360(2) (2015) 213–8. [DOI] [PubMed] [Google Scholar]
- [31].Hong MKH, Macintyre G, Wedge DC, Loo PV, Patel K, Lunke S, Alexandrov LB, Sloggett C, Cmero M, Marass F, Tsui D, Mangiola S, Lonie A, Naeem H, Sapre N, Phal PM, Kurganovs N, Chin X, Kerger M, Warren AY, Neal D, Gnanapragasam V, Rosenfeld N, Pedersen JS, Ryan A, Haviv I, Costello AJ, Corcoran NM, Hovens CM, Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer, Nature Communications 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M, Holland-Letz T, Hofner T, Sprick M, Scharpff M, Marme F, Sinn HP, Pantel K, Weichert W, Trumpp A, Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay, Nat Biotechnol 31(6) (2013) 539–44. [DOI] [PubMed] [Google Scholar]
- [33].Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, Ward T, Blackhall FH, Dive C, Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer, J Clin Oncol 30(5) (2012) 525–32. [DOI] [PubMed] [Google Scholar]
- [34].Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S, Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis, Cell 158(5) (2014) 1110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cheung KJ, Gabrielson E, Werb Z, Ewald AJ, Collective invasion in breast cancer requires a conserved basal epithelial program, Cell 155(7) (2013) 1639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Westcott JM, Prechtl AM, Maine EA, Dang TT, Esparza MA, Sun H, Zhou Y, Xie Y, Pearson GW, An epigenetically distinct breast cancer cell subpopulation promotes collective invasion, J Clin Invest 125(5) (2015) 1927–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rack B, Juckstock J, Gunthner-Biller M, Andergassen U, Neugebauer J, Hepp P, Schoberth A, Mayr D, Zwingers T, Schindlbeck C, Friese K, Janni W, Trastuzumab clears HER2/neu-positive isolated tumor cells from bone marrow in primary breast cancer patients, Arch Gynecol Obstet 285(2) (2012) 485–92. [DOI] [PubMed] [Google Scholar]
- [38].de Albuquerque A, Kubisch I, Ernst D, Breier G, Stamminger G, Fersis N, Stolzel U, Boese-Landgraf J, Eichler A, Kaul S, Development of a molecular multimarker assay for the analysis of circulating tumor cells in adenocarcinoma patients, Clinical laboratory 58(5–6) (2012) 373–84. [PubMed] [Google Scholar]
- [39].Fehm T, Krawczyk N, Solomayer EF, Becker-Pergola G, Durr-Storzer S, Neubauer H, Seeger H, Staebler A, Wallwiener D, Becker S, ERalpha-status of disseminated tumour cells in bone marrow of primary breast cancer patients, Breast Cancer Res 10(5) (2008) R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, Brewer DS, Kallio HML, Hognas G, Annala M, Kivinummi K, Goody V, Latimer C, O’Meara S, Dawson KJ, Isaacs W, Emmert-Buck MR, Nykter M, Foster C, Kote-Jarai Z, Easton D, Whitaker HC, Neal DE, Cooper CS, Eeles RA, Visakorpi T, Campbell PJ, McDermott U, Wedge DC, Bova GS, The evolutionary history of lethal metastatic prostate cancer, Nature 520(7547) (2015) 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sanborn JZ, Chung J, Purdom E, Wang NJ, Kakavand H, Wilmott JS, Butler T, Thompson JF, Mann GJ, Haydu LE, Saw RPM, Busam KJ, Lo RS, Collisson EA, Hur JS, Spellman PT, Cleaver JE, Gray JW, Huh N, Murali R, Scolyer RA, Bastian BC, Cho RJ, Phylogenetic analyses of melanoma reveal complex patterns of metastatic dissemination, Proceedings of the National Academy of Sciences 112(35) (2015) 10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Maddipati R, Stanger BZ, Pancreatic Cancer Metastases Harbor Evidence of Polyclonality, Cancer discovery 5(10) (2015) 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta KJ, Bader JS, Ewald AJ, Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters, Proc Natl Acad Sci U S A 113(7) (2016) E854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reeves MQ, Kandyba E, Harris S, Del Rosario R, Balmain A, Multi-color lineage tracing reveals clonal dynamics of cancer evolution from initiation to metastasis, Nat Cell Biol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Friedl P, Locker J, Sahai E, Segall JE, Classifying collective cancer cell invasion, Nat Cell Biol 14(8) (2012) 777–83. [DOI] [PubMed] [Google Scholar]
- [46].Friedl P, Noble PB, Walton PA, Laird DW, Chauvin PJ, Tabah RJ, Black M, Zanker KS, Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro, Cancer Res 55(20) (1995) 4557–60. [PubMed] [Google Scholar]
- [47].Veracini L, Grail D, Schaub S, Beghelli-de la Forest Divonne S, Etienne-Grimaldi MC, Milano G, Bozec A, Babin E, Sudaka A, Thariat J, Van Obberghen-Schilling E, Elevated Src family kinase activity stabilizes E-cadherin-based junctions and collective movement of head and neck squamous cell carcinomas, Oncotarget 6(10) (2015) 7570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chung YC, Wei WC, Hung CN, Kuo JF, Hsu CP, Chang KJ, Chao WT, Rab11 collaborates E-cadherin to promote collective cell migration and indicates a poor prognosis in colorectal carcinoma, European journal of clinical investigation 46(12) (2016) 1002–1011. [DOI] [PubMed] [Google Scholar]
- [49].Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA, Distinct EMT programs control normal mammary stem cells and tumour-initiating cells, Nature 525(7568) (2015) 256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K, Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity, Nature (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Janiszewska M, Tabassum DP, Castaño Z, Cristea S, Yamamoto KN, Kingston NL, Murphy KC, Shu S, Harper NW, C. GDA, Aleckovic M, Ekram M, Cohen O, Kwak M, Qin T, Laszewski T, Luoma A, Marusyk A, Wucherpfennig K, Wagle N, Fan R, Michor F, McAllister F, Polyak K, Subclonal cooperation drives metastasis by modulating local and systemic immune microenvironments, Nature Cell Biol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mateo F, Meca-Cortes O, Celia-Terrassa T, Fernandez Y, Abasolo I, Sanchez-Cid L, Bermudo R, Sagasta A, Rodriguez-Carunchio L, Pons M, Canovas V, Marin-Aguilera M, Mengual L, Alcaraz A, Schwartz S Jr., Mellado B, Aguilera KY, Brekken R, Fernandez PL, Paciucci R, Thomson TM, SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations, Mol Cancer 13(2014) 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L, Massagué J, Tumor self-seeding by circulating cancer cells, Cell 139(7) (2009) 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJH, Witt H, Croul S, Bouffet E, Fults DW, Eberhart CG, Garzia L, Meter TV, Zagzag D, Jabado N, Schwartzentruber J, Majewski J, Scheetz TE, Pfister SM, Korshunov A, Li X-N, Scherer SW, Cho Y-J, Akagi K, Macdonald TJ, Koster J, Mccabe MG, Sarver AL, Collins VP, Weiss WA, Largaespada DA, Collier LS, Taylor MD, Clonal selection drives genetic divergence of metastatic medulloblastoma, Nature 482(7386) (2012) 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Almendro V, Kim HJ, Cheng YK, Gonen M, Itzkovitz S, Argani P, van Oudenaarden A, Sukumar S, Michor F, Polyak K, Genetic and phenotypic diversity in breast tumor metastases, Cancer Res 74(5) (2014) 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, Soria JC, Dien AT, Adnani Y, Kamal M, Gamier S, Meurice G, Jimenez M, Dogan S, Verret B, Chaffanet M, Bachelot T, Campone M, Lefeuvre C, Bonnefoi H, Dalenc F, Jacquet A, De Filippo MR, Babbar N, Birnbaum D, Filleron T, Le Tourneau C, Andre F, Genomic characterization of metastatic breast cancers, Nature 569(7757) (2019) 560–564. [DOI] [PubMed] [Google Scholar]
- [57].Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, lacobuzio-Donahue C, Futreal PA, The patterns and dynamics of genomic instability in metastatic pancreatic cancer, Nature 467(7319) (2010) 1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shukla A, Alsarraj J, Hunter K, Understanding susceptibility to breast cancer metastasis: the genetic approach, Breast cancer management 3(2) (2014) 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guy CT, Cardiff RD, Muller WJ, Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease, Mol Cell Biol 12(3) (1992) 954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Martin MD, Carter KJ, Jean-Philippe SR, Chang M, Mobashery S, Thiolloy S, Lynch CC, Matrisian LM, Fingleton B, Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background, Cancer Res 68(15) (2008) 6251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Takahashi M, Dinse GE, Foley JF, Hardisty JF, Maronpot RR, Comparative prevalence, multiplicity, and progression of spontaneous and vinyl carbarn ate-induced liver lesions in five strains of male mice, Toxicol Pathol 30(5) (2002) 599–605. [DOI] [PubMed] [Google Scholar]
- [62].Rohan RM, Fernandez A, Udagawa T, Yuan J, D’Amato RJ, Genetic heterogeneity of angiogenesis in mice, Faseb J 14(7) (2000) 871–6. [DOI] [PubMed] [Google Scholar]
- [63].Zhu S, Zhang X, Weichert-Leahey N, Dong Z, Zhang C, Lopez G, Tao T, He S, Wood AC, Oldridge D, Ung CY, van Ree JH, Khan A, Salazar BM, Lummertz da Rocha E, Zimmerman MW, Guo F, Cao H, Hou X, Weroha SJ, Perez-Atayde AR, Neuberg DS, Meves A, McNiven MA, van Deursen JM, Li H, Maris JM, Look AT, LM01 Synergizes with MYCN to Promote Neuroblastoma Initiation and Metastasis, Cancer Cell 32(3) (2017) 310–323.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D, Pre-metastatic niches: organ-specific homes for metastases, Nat Rev Cancer 17(5) (2017) 302–317. [DOI] [PubMed] [Google Scholar]
- [65].Aleckovic M, Kang Y, Regulation of cancer metastasis by cell-free miRNAs, Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1855(1) (2015) 24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Jørgen Labori K, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature 527(7578) (2015) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gao D, Mittal V, The role of bone-marrow-derived cells in tumor growth, metastasis initiation and progression, Trends in Molecular Medicine 15(8) (2009) 333–343. [DOI] [PubMed] [Google Scholar]
- [68].Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M, MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis, Cancer Cell 2(4) (2002) 289–300. [DOI] [PubMed] [Google Scholar]
- [69].Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D, VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche, Nature 438(7069) (2005) 820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hiratsuka S, Watanabe A, Aburatani H, Maru Y, Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis, Nat Cell Biol 8(12) (2006) 1369–75. [DOI] [PubMed] [Google Scholar]
- [71].Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le Q-T, Chi J-TA, Jeffrey SS, Giaccia AJ, Lysyl oxidase is essential for hypoxia-induced metastasis, Nature 440(7088) (2006) 1222–1226. [DOI] [PubMed] [Google Scholar]
- [72].Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K, Molecular characterization of the tumor microenvironment in breast cancer, Cancer Cell 6(1) (2004) 17–32. [DOI] [PubMed] [Google Scholar]
- [73].Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA, Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion, Cell 121(3) (2005) 335–48. [DOI] [PubMed] [Google Scholar]
- [74].Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar CM, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D, Melanoma exosomes educate bone marrow progenitor cells toward a prometastatic phenotype through MET, Nat Med (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Obenauf AC, Massague J, Surviving at a Distance: Organ-Specific Metastasis, Trends Cancer 1 (1) (2015) 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massague J, Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis, Cell 168(6) (2017) 1101–1113.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].van der Weyden L, Arends MJ, Campbell AD, Bald T, Wardle-Jones H, Griggs N, Velasco-Herrera MD, Tuting T, Sansom OJ, Karp NA, Clare S, Gleeson D, Ryder E, Galli A, Tuck E, Cambridge EL, Voet T, Macaulay IC, Wong K, Spiegel S, Speak AO, Adams DJ, Genome-wide in vivo screen identifies novel host regulators of metastatic colonization, Nature 541 (7636) (2017) 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL, Abrogation of TGFb Signaling in Mammary Carcinomas Recruits Gr-1+CD11b+ Myeloid Cells that Promote Metastasis, Cancer Cell (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L, Gr-1+CD11b+ Myeloid Cells Tip the Balance of Immune Protection to Tumor Promotion in the Premetastatic Lung, Cancer research 70(15) (2010) 6139–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Brüggen N, Peale FV, Carano R, Meng YG, Ferrara N, Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes, Proc Natl Acad Sei U S A 107(50) (2010) 21248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Serafini P, Borrello I, Bronte V, Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression, Seminars in cancer biology 16(1) (2006) 53–65. [DOI] [PubMed] [Google Scholar]
- [82].Almand B, Clark J.l., Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI, Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer, J Immunol 166(1) (2001) 678–89. [DOI] [PubMed] [Google Scholar]
- [83].Coffelt SB, de Visser KE, Systemic inflammation: Cancer’s long-distance reach to maximize metastasis, Oncoimmunology 5(2) (2016) e1075694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kersten K, Coffelt SB, Hoogstraat M, Verstegen NJM, Vrijland K, Ciampricotti M, Doornebal CW, Hau C-S, Wellenstein MD, Salvagno C, Doshi P, Lips EH, Wessels LFA, de Visser KE, Mammary tumor-derived CCL2 enhances prometastatic systemic inflammation through upregulation of IL1 ß in tumor-associated macrophages, Oncoimmunology 6(8) (2017) e1334744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lu X, Kang Y, Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone, J Biol Chem 284(42) (2009) 29087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW, CCL2 recruits inflammatory monocytes to facilitate breast tumor metastasis, Nature 475(7355) (2011) 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Voloshin T, Alishekevitz D, Kaneti L, Miller V, Isakov E, Kaplanov I, Voronov E, Fremder E, Benhar M, Machluf M, Apte RN, Shaked Y, Blocking IL1 ß Pathway Following Paclitaxel Chemotherapy Slightly Inhibits Primary Tumor Growth but Promotes Spontaneous Metastasis, Molecular Cancer Therapeutics 14(6) (2015) 1385–1394. [DOI] [PubMed] [Google Scholar]
- [88].Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, Nakasone ES, Hearn SA, Kuttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg MS, Egeblad M, Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps, Sei Transl Med 8(361) (2016) 361 ra 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, de Visser KE, IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis, Nature 522(7556) (2015) 345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, Nakano K, Tsuboi M, Shibata K, Furuse K, Fukushima M, Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00–03, Eur J Cancer 45(11) (2009) 1950–8. [DOI] [PubMed] [Google Scholar]
- [91].An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ, Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer, Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals 15(6) (2010) 516–22. [DOI] [PubMed] [Google Scholar]
- [92].Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K, Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment, Cancer Immunol Immunother 58(1) (2009) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T, Evaluations of interferon-y/inter leu kin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients, Journal of Surgical Oncology 102(7) (2010) 742–747. [DOI] [PubMed] [Google Scholar]
- [94].Liu H, Liu G, Bao Q, Sun W, Bao H, Bi L, Wen W, Liu Y, Wang Z, Yin X, Bai Y, Hu X, The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in rectal carcinoma, Journal of gastrointestinal cancer 41 (2) (2010) 116–20. [DOI] [PubMed] [Google Scholar]
- [95].Atzpodien J, Reitz M, Peripheral blood neutrophils as independent immunologic predictor of response and long-term survival upon immunotherapy in metastatic renalcell carcinoma, Cancer Biother Radiopharm 23(1) (2008) 129–34. [DOI] [PubMed] [Google Scholar]
- [96].Hasegawa S, Suda T, Negi K, Hattori Y, Lung large cell carcinoma producing granulocyte-colony-stimulating factor, Ann Thorac Surg 83(1) (2007) 308–10. [DOI] [PubMed] [Google Scholar]
- [97].Granger JM, Kontoyiannis DP, Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study, Cancer 115(17) (2009) 3919–23. [DOI] [PubMed] [Google Scholar]
- [98].Wu TC, Xu K, Martinek J, Young RR, Banchereau R, George J, Turner J, Kim KI, Zurawski S, Wang X, Blankenship D, Brookes HM, Marches F, Obermoser G, Lavecchio E, Levin MK, Bae S, Chung CH, Smith JL, Cepika AM, Oxley KL, Snipes GJ, Banchereau J, Pascual V, O’Shaughnessy J, Palucka AK, IL1 Receptor Antagonist Controls Transcriptional Signature of Inflammation in Patients with Metastatic Breast Cancer, Cancer Res 78(18) (2018) 5243–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R, Tumor entrained neutrophils inhibit seeding in the premetastatic lung, Cancer Cell 20(3) (2011) 300–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S, Yang SR, Kurian A, Van Valen D, West R, Bendall SC, Angelo M, A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging, Cell 174(6) (2018) 1373–1387 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gruosso T, Gigoux M, Manem VSK, Bertos N, Zuo D, Perlitch I, Saleh SMI, Zhao H, Souleimanova M, Johnson RM, Monette A, Ramos VM, Hallett MT, Stagg J, Lapointe R, Omeroglu A, Meterissian S, Buisseret L, Van den Eynden G, Salgado R, Guiot MC, Haibe-Kains B, Park M, Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers, J Clin Invest 129(4) (2019) 1785–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, Krummel MF, Visualization of immediate immune responses to pioneer metastatic cells in the lung, Nature 531(7595) (2016) 513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sharma NL, Massie CE, Ramos-Montoya A, Zecchini V, Scott HE, Lamb AD, MacArthur S, Stark R, Warren AY, Mills IG, Neal DE, The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man, Cancer Cell 23(1) (2013) 35–47. [DOI] [PubMed] [Google Scholar]
- [104].Labelle M, Begum S, Hynes RO, Platelets guide the formation of early metastatic niches, Proc Natl Acad Sci U S A 111 (30) (2014) E3053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kuznetsov HS, Marsh T, Markens BA, Castano Z, Greene-Colozzi A, Hay SA, Brown VE, Richardson AL, Signoretti S, Battinelli EM, McAllister SS, Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells, Cancer Discov 2(12) (2012) 1150–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA, Systemic endocrine instigation of indolent tumor growth requires osteopontin, Cell 133(6) (2008) 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, Carpenter AE, Jirstrom K, Magnusson K, Ebert BL, Ponten F, Weinberg RA, McAllister SS, Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice, J Clin Invest 121(2) (2011)784–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, Engle D, Campbell F, Palmer D, Ko JH, Tuveson DA, Hirsch E, Mielgo A, Schmid MC, Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis, Nat Cell Biol 18(5) (2016) 549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bartoschek M, Oskolkov N, Bocci M, Lovrot J, Larsson C, Sommarin M, Madsen CD, Lindgren D, Pekar G, Karlsson G, Ringner M, Bergh J, Bjorklund A, Pietras K, Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing, Nat Commun 9(1) (2018) 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Costa A, Kieffer Y, Scholer-Dahirel A, Pelón F, Bourachot B, Cardón M, Sirven P, Magagna I, Fuhrmann L, Bernard C, Bonneau C, Kondratova M, Kuperstein I, Zinovyev A, Givel AM, Parrini MC, Soumelis V, Vincent-Salomon A, Mechta-Grigoriou F, Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer, Cancer Cell 33(3) (2018) 463–479 e10. [DOI] [PubMed] [Google Scholar]
- [111].Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D, Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth, Nature 527(7576) (2015) 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chen Q, Sun L, Chen ZJ, Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing, Nat Immunol 17(10) (2016) 1142–9. [DOI] [PubMed] [Google Scholar]
- [113].Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, Doglio L, Martínez L, Martínez-Saez E, Cajal S.R.y., Megías D, Hernández-Encinas E, Blanco-Aparicio C, Martínez L, Zarzuela E, Muñoz J, Fustero-Torre C, Piñeiro-Yáñez E, Hernández-Laín A, Bertero L, Poli V, Sanchez-Martinez M, Menendez JA, Soffietti R, Bosch-Barrera J, Valiente M, STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis, Nature Medicine 24(7) (2018) 1024–1035. [DOI] [PubMed] [Google Scholar]
- [114].Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, Griscom B, Rosenblum M, Boire A, Brogi E, Giancotti FG, Schachner M, Malladi S, Massagué J, Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization, Nature cell biology 20(8) (2018) 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Luo X, Fu Y, Loza AJ, Murali B, Leahy KM, Ruhland MK, Gang M, Su X, Zamani A, Shi Y, Lavine KJ, Ornitz DM, Weilbaecher KN, Long F, Novack DV, Faccio R, Longmore GD, Stewart SA, Stromal-lnitiated Changes in the Bone Promote Metastatic Niche Development, Cell reports 14(1) (2016) 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Murali B, Ren Q, Luo X, Faget DV, Wang C, Johnson RM, Gruosso T, Flanagan KC, Fu Y, Leahy K, Alspach E, Su X, Ross MH, Burnette B, Weilbaecher KN, Park M, Mbalaviele G, Monahan JB, Stewart SA, Inhibition of the Stromal p38MAPK/MK2 Pathway Limits Breast Cancer Metastases and Chemotherapy-Induced Bone Loss, Cancer Res 78(19) (2018) 5618–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH 3rd, Dang VM, Appleton J, O’Connell MP, Cheng P, Valiga AA, Morissette R, McDonnell NB, Ferrucci L, Kossenkov AV, Meeth K, Tang HY, Yin X, Wood WH 3rd, Lehrmann E, Becker KG, Flaherty KT, Frederick DT, Wargo JA, Cooper ZA, Tetzlaff MT, Hudgens C, Aird KM, Zhang R, Xu X, Liu Q, Bartlett E, Karakousis G, Eroglu Z, Lo RS, Chan M, Menzies AM, Long GV, Johnson DB, Sosman J, Schilling B, Schadendorf D, Speicher DW, Bosenberg M, Ribas A, Weeraratna AT, sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance, Nature 532(7598) (2016) 250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Marsh T, Wong I, Sceneay J, Barakat A, Qin Y, Sjodin A, Alspach E, Nilsson B, Stewart SA, McAllister SS, Hematopoietic Age at Onset of Triple-Negative Breast Cancer Dictates Disease Aggressiveness and Progression, Cancer Res 76(10) (2016) 2932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Liedtke C, Rody A, Gluz O, Baumann K, Beyer D, Kohls EB, Lausen K, Hanker L, Holtrich U, Becker S, Karn T, The prognostic impact of age in different molecular subtypes of breast cancer, Breast Cancer Res Treat 152(3) (2015) 667–73. [DOI] [PubMed] [Google Scholar]
- [120].Calle EE, Kaaks R, Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms, Nat Rev Cancer 4(8) (2004) 579–91. [DOI] [PubMed] [Google Scholar]
- [121].Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S, Effect of obesity on prognosis after early-stage breast cancer, J Clin Oncol 29(1) (2011) 25–31. [DOI] [PubMed] [Google Scholar]
- [122].Osman MA, Hennessy BT, Obesity Correlation With Metastases Development and Response to First-Line Metastatic Chemotherapy in Breast Cancer, Clinical Medicine Insights. Oncology 9 (2015) 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Quail DF, Olson OC, Bhardwaj P, Walsh LA, Akkari L, Quick ML, Chen IC, Wendel N, Ben-Chetrit N, Walker J, Holt PR, Dannenberg AJ, Joyce JA, Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF, Nat Cell Biol 19(8) (2017) 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, O’Farrelly C, Raverdeau M, Vernon A, Pettee W, O’Shea D, Nikolajczyk BS, Mills KHG, Brenner MB, Finlay D, Lynch L, Metabolic reprogramming of natural killer cells in obesity limits antitumor responses, Nature Immunology 19(12) (2018) 1330–1340. [DOI] [PubMed] [Google Scholar]
- [125].McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee D-Y, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang S, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob J-J, Flaherty KT, Walker D, Yan Y, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA, Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis, The Lancet. Oncology 19(3) (2018) 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang Z, Aguilar EG, Luna J.l., Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, Grossenbacher SK, Withers SS, Rebhun RB, Hartigan-O’Connor DJ, Mendez-Lagares G, Tarantal AF, Isseroff RR, Griffith TS, Schalper KA, Merleev A, Saha A, Maverakis E, Kelly K, Aljumaily R, Ibrahimi S, Mukherjee S, Machiorlatti M, Vesely SK, Longo DL, Blazar BR, Canter RJ, Murphy WJ, Monjazeb AM, Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade, Nature Medicine (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Rubio-Patino C, Bossowski JP, De Donatis GM, Mondragon L, Villa E, Aira LE, Chiche J, Mhaidly R, Lebeaupin C, Marchetti S, Voutetakis K, Chatziioannou A, Castelli FA, Lamourette P, Chu-Van E, Fenaille F, Avril T, Passeron T, Patterson JB, Verhoeyen E, Bailly-Maitre B, Chevet E, Ricci JE, Low-Protein Diet Induces IREIalpha-Dependent Anticancer Immunosurveillance, Cell Metab 27(4) (2018) 828–842.e7. [DOI] [PubMed] [Google Scholar]
- [128].Hardee JP, Porter RR, Sui X, Archer E, Lee IM, Lavie CJ, Blair SN, The effect of resistance exercise on all-cause mortality in cancer survivors, Mayo Clinic proceedings 89(8) (2014) 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Schmid D, Leitzmann MF, Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis, Ann Oncol 25(7) (2014) 1293–311. [DOI] [PubMed] [Google Scholar]
- [130].Li Y, Gu M, Jing F, Cai S, Bao C, Wang J, Jin M, Chen K, Association between physical activity and all cancer mortality: Dose-response meta-analysis of cohort studies, International Journal of Cancer 138(4) (2016) 818–832. [DOI] [PubMed] [Google Scholar]
- [131].Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, Nielsen J, Gehl J, Pedersen BK, Thor Straten P, Hojman P, Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution, Cell Metab 23(3) (2016) 554–62. [DOI] [PubMed] [Google Scholar]
- [132].Berrueta L, Bergholz J, Munoz D, Muskaj I, Badger GJ, Shukla A, Kim HJ, Zhao JJ, Langevin HM, Stretching Reduces Tumor Growth in a Mouse Breast Cancer Model, Scientific Reports 8(1) (2018) 7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, Kuperwasser C, Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers, Cancer Res 67(5) (2007) 2062–71. [DOI] [PubMed] [Google Scholar]
- [134].Iyer V, Klebba I, McCready J, Arendt LM, Betancur-Boissel M, Wu MF, Zhang X, Lewis MT, Kuperwasser C, Estrogen promotes ER-negative tumor growth and angiogenesis through mobilization of bone marrow-derived monocytes, Cancer Res 72(11) (2012) 2705–13. [DOI] [PubMed] [Google Scholar]
- [135].Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P, Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression, Int J Cancer 136(8) (2015) 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Betts CB, Pennock ND, Caruso BP, Ruffell B, Borges VF, Schedin P, Mucosal Immunity in the Female Murine Mammary Gland, J Immunol 201(2) (2018) 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Amant F, von Minckwitz G, Han SN, Bontenbal M, Ring AE, Giermek J, Wildiers H, Fehm T, Linn SC, Schlehe B, Neven P, Westenend PJ, Muller V, Van Calsteren K, Rack B, Nekljudova V, Harbeck N, Untch M, Witteveen PO, Schwedler K, Thomssen C, Van Calster B, Loibl S, Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study, J Clin Oncol 31 (20) (2013) 2532–9. [DOI] [PubMed] [Google Scholar]
- [138].Pennock ND, Martinson HA, Guo Q, Betts CB, Jindal S, Tsujikawa T, Coussens LM, Borges VF, Schedin P, Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer, Journal for immunotherapy of cancer 6(1) (2018) 98–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Taranova AG, Maldonado D 3rd, Vachon CM, Jacobsen EA, Abdala-Valencia H, McGarry MP, Ochkur SI, Protheroe CA, Doyle A, Grant CS, Cook-Mills J, Birnbaumer L, Lee NA, Lee JJ, Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung, Cancer Res 68(20) (2008) 8582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Roy LD, Ghosh S, Pathangey LB, Tinder TL, Gruber HE, Mukherjee P, Collagen induced arthritis increases secondary metastasis in MMTV-PyV MT mouse model of mammary cancer, BMC Cancer 11 (2011) 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Brembilla NC, Senra L, Boehncke W-H, The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond, Frontiers in Immunology 9(1682) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, Lambert AW, Bierie B, Ploegh HL, Dougan SK, Weinberg RA, The systemic response to surgery triggers the outgrowth of distant immune-control led tumors in mouse models of dormancy, Science Translational Medicine 10(436) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, Massague J, Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT, Cell 165(1) (2016) 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gawrzak S, Rinaldi L, Gregorio S, Arenas EJ, Salvador F, Urosevic J, Figueras-Puig C, Rojo F, Del Barco Barrantes I, Cejalvo JM, Palafox M, Guiu M, Berenguer-Llergo A, Symeonidi A, Bellmunt A, Kalafatovic D, Arnal-Estape A, Fernandez E, Mullauer B, Groeneveld R, Slobodnyuk K, Stephan-Otto Attolini C, Saura C, Arribas J, Cortes J, Rovira A, Munoz M, Lluch A, Serra V, Albanell J, Prat A, Nebreda AR, Benitah SA, Gomis RR, MSK1 regulates luminal cell differentiation and metastatic dormancy in ER(+) breast cancer, Nat Cell Biol 20(2) (2018) 211–221. [DOI] [PubMed] [Google Scholar]
- [145].Rueda OM, Sammut SJ, Seoane JA, Chin SF, Caswell-Jin JL, Callari M, Batra R, Pereira B, Bruna A, Ali HR, Provenzano E, Liu B, Parisien M, Gillett C, McKinney S, Green AR, Murphy L, Purushotham A, Ellis IO, Pharoah PD, Rueda C, Aparicio S, Caldas C, Curtis C, Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups, Nature 567(7748) (2019) 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sosa MS, Bragado P, Aguirre-Ghiso JA, Mechanisms of disseminated cancer cell dormancy: an awakening field, Nature Reviews Cancer 14 (2014) 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Alečković M, Kang Y, Bone marrow stroma-derived miRNAs as regulators, biomarkers and therapeutic targets of bone metastasis, BoneKEy Rep 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Duffy MJ, Use of Biomarkers in Screening for Cancer, in: Scatena R (Ed.), Advances in Cancer Biomarkers: From biochemistry to clinic for a critical revision, Springer; Netherlands, Dordrecht, 2015, pp. 27–39. [Google Scholar]
- [149].Leary RJ, Sausen M, Kinde I, Papadopoulos N, Carpten JD, Craig D, O’Shaughnessy J, Kinzler KW, Parmigiani G, Vogelstein B, Diaz LA Jr., Velculescu VE, Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing, Sci Transl Med 4(162) (2012) 162ra154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R, Glypican-1 identifies cancer exosomes and detects early pancreatic cancer, Nature 523(7559) (2015) 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].McMillan DC, The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer, Cancer Treat Rev 39(5) (2013) 534–40. [DOI] [PubMed] [Google Scholar]
- [152].Shojaei F, Scott N, Kang X, Lappin PB, Fitzgerald AA, Karlicek S, Simmons BH, Wu A, Lee JH, Bergqvist S, Kraynov E, Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer, Journal of experimental & clinical cancer research : CR 31 (2012) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cheung ST, Wong SY, Leung KL, Chen X, So S, Ng IO, Fan ST, Granulin-epithelin precursor overexpression promotes growth and invasion of hepatocellular carcinoma, Clin Cancer Res 10(22) (2004) 7629–36. [DOI] [PubMed] [Google Scholar]
- [154].Ubellacker JM, Haider MT, DeCristo MJ, Allocca G, Brown NJ, Silver DP, Holen I, McAllister SS, Zoledronic acid alters hematopoiesis and generates breast tumor-suppressive bone marrow cells, Breast Cancer Res 19(1) (2017) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Ubellacker JM, Baryawno N, Severe N, DeCristo MJ, Sceneay J, Hutchinson JN, Haider MT, Rhee CS, Qin Y, Gregory WM, Garrido-Castro AC, Holen I, Brown JE, Coleman RE, Scadden DT, McAllister SS, Modulating Bone Marrow Hematopoietic Lineage Potential to Prevent Bone Metastasis in Breast Cancer, Cancer Res 78(18) (2018) 5300–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE, Aspirin intake and survival after breast cancer, J Clin Oncol 28(9) (2010) 1467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen J.l., Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU, A randomized trial of aspirin to prevent colorectal adenomas, N Engl J Med 348(10) (2003) 891–9. [DOI] [PubMed] [Google Scholar]
- [158].Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, Sun R, Nosho K, Meyerhardt JA, Giovannucci E, Fuchs CS, Chan AT, Ogino S, Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival, N Engl J Med 367(17) (2012) 1596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Johnson KE, Forward JA, Tippy MD, Ceglowski JR, El-Husayni S, Kulenthirarajan R, Machlus KR, Mayer EL, Italiano JE Jr., Battinelli EM, Tamoxifen Directly Inhibits Platelet Angiogenic Potential and Platelet-Mediated Metastasis, Arterioscler Thromb Vase Biol 37(4) (2017) 664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Voloshin T, Alishekevitz D, Kaneti L, Miller V, Isakov E, Kaplanov I, Voronov E, Fremder E, Benhar M, Machluf M, Apte RN, Shaked Y, Blocking ILIbeta Pathway Following Paclitaxel Chemotherapy Slightly Inhibits Primary Tumor Growth but Promotes Spontaneous Metastasis, Mol Cancer Ther 14(6) (2015) 1385–94. [DOI] [PubMed] [Google Scholar]