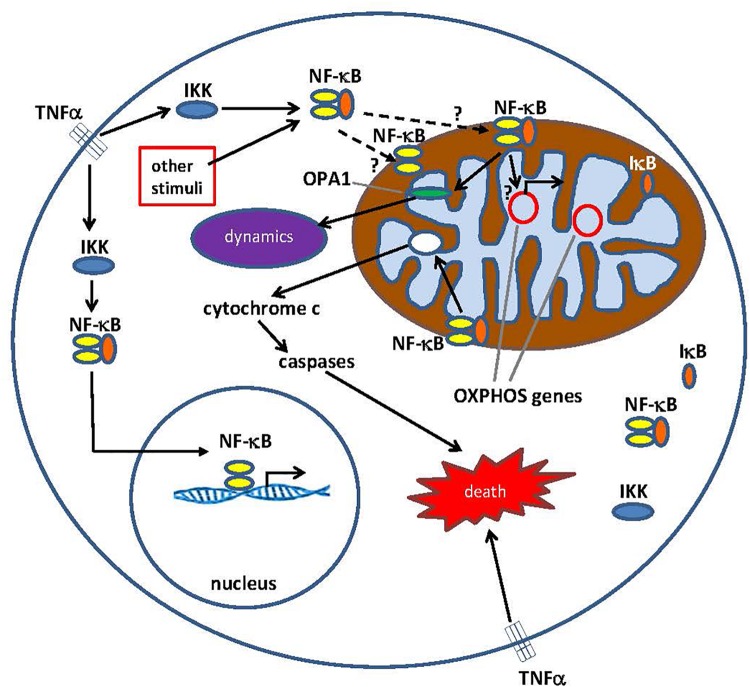
FIGURE 1.
Pathways for Nuclear factor κ B (NF-κB) signaling in the cytoplasm and the mitochondrion. The NF-κB tri-subunt complex (e.g., p65, p50, IκB – one possible combination) exists in an inactive state in the cytoplasm. NF-κB activation is initiated when molecules such as TNFα bind to TNF receptors (different types exist). Other external or internal stimuli can also activate NF-κB. A complicated signal transduction process then begins once TNF receptors are activated; IκB kinase (IKK) is ultimately triggered and leads to the phosphorylation of IκB, which results in IκB ubiquitination and degradation. Once IκB is degraded, the remaining NF-κB dimer (e.g., p65/p50 or p50/p50 subunit combinations are possible) translocates to the nucleus, where it binds to a DNA consensus sequence of target genes. By processes not well understood, the NF-κB complex or NF-κB subunits can also migrate into the mitochondrion, where evidence suggests it/they occupies the intermembrane space. Once inside the mitochondria, NF-κB is thought to interact with OXPHOS genes (mitochondrial mtDNA) that leads to the expression of proteins involved in various functions, including mitochondrial dynamics and COX III regulation (component of Complex IV). Evidence also suggests, NF-κB can function as a switch in the mitochondria and control the balance between the utilization of cytoplasmic glycolysis and mitochondrial respiration in normal cells and in cancer. Finally, data also point to intrinsic apoptotic pathway stimulation, where NF-κB activation in the mitochondria leads to cytochrome c release, thus triggering caspase cascades and programed cell death.