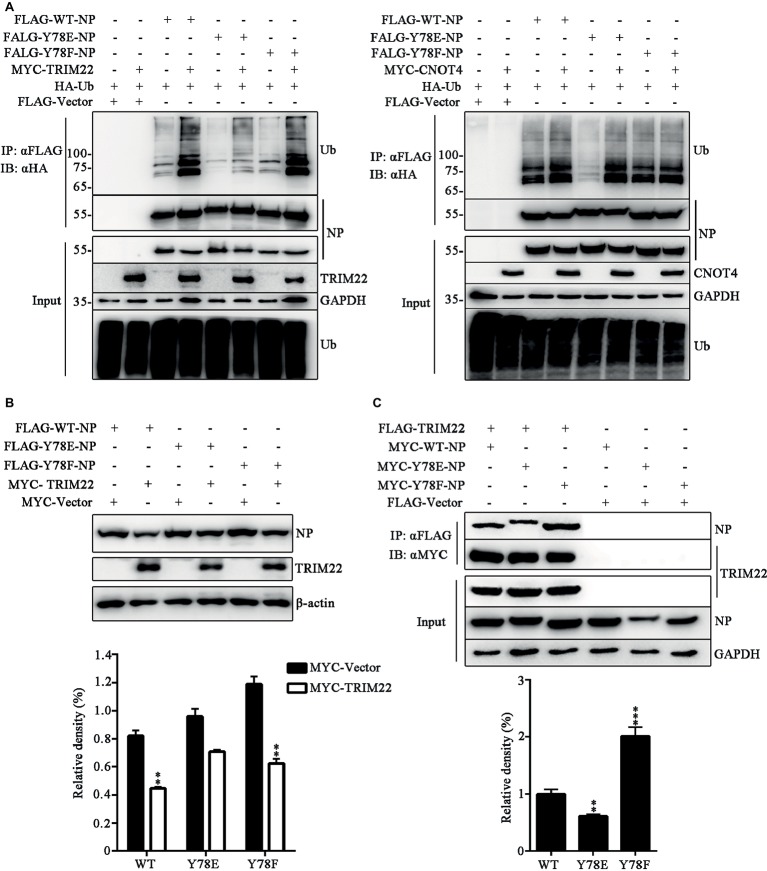
Figure 8.
Y78 phosphorylation regulates TRIM22-mediated NP ubiquitination. (A) Effect of TRIM22 and CNOT4 on the ubiquitination of NPs. Immunoblot analysis of lysates in 293 T cells transfected with various combinations of plasmids for 30 h, followed by immunoprecipitation with anti-FLAG beads. (B) Effect of TRIM22 on the stability of IAV NPs. 293 T cells were transfected with FLAG-NP (WT, Y78F, or Y78E) plasmid, along with MYC-TRIM22 or an empty vector for 30 h. Cells were lysed and then detected with corresponding antibodies (top). The relative density of NPs was normalized to β-actin (below). Data are shown as mean + SD (n = 3). Differences of NP stability between the TRIM22- and Vector-transfected groups were tested using unpaired Student’s t-test. **p < 0.01. (C) Effect of Y78 phosphorylation on the interaction of TRIM22 with WT or mutant NPs. 293 T cells were transfected with MYC-NP (WT, Y78F, and Y78E) and FLAG-TRIM22 and lysed at 30 h.p.t. FLAG-TRIM22 was immunoprecipitated with anti-FLAG beads, and the associated NPs were detected using rabbit anti-NP antibody (top). The relative density of immunoprecipitated NPs was normalized to immunoprecipitated TRIM22 (below). Data are shown as mean + SD (n = 3). Differences between mutant and WT NPs were evaluated using one-way ANOVA followed by Dunnett’s test. **p < 0.01; ***p < 0.001.