Abstract
Background
Pregnant women have an elevated risk of illness and hospitalisation from influenza. Pregnant women are recommended to be prioritised for influenza vaccination during any stage of pregnancy. The risk of seasonal influenza varies substantially throughout the year in temperate climates; however, there is limited knowledge of how vaccination timing during pregnancy impacts the benefits received by the mother and foetus.
Objectives
To compare antenatal vaccination timing with regard to influenza vaccine immunogenicity during pregnancy and transplacental transfer to their newborns.
Methods
Studies were eligible for inclusion if immunogenicity to influenza vaccine was evaluated in women stratified by trimester of pregnancy. Haemagglutination inhibition (HI) titres, stratified by trimester of vaccination, had to be measured at either pre‐vaccination and within one month post‐vaccination, post‐vaccination and at delivery in the mother, or in cord/newborn blood. Authors searched PubMed, Scopus, Web of Science and EMBASE databases from inception until June 2016 and authors of identified studies were contacted for additional data. Extracted data were tabulated and summarised via random‐effect meta‐analyses and qualitative methods.
Results
Sixteen studies met the inclusion criteria. Meta‐analyses found that compared with women vaccinated in an earlier trimester, those vaccinated in a later trimester had a greater fold increase in HI titres (1.33‐ to 1.96‐fold) and higher HI titres in cord/newborn blood (1.21‐ to 1.64‐fold).
Conclusions
This review provides comparative analysis of the effect of vaccination timing on maternal immunogenicity and protection of the infant that is informative and relevant to current vaccine scheduling for pregnant women.
Keywords: immunogenicity, influenza, pregnancy, timing, trimester, vaccination
1. INTRODUCTION
Pregnant women have a particularly high risk of illness and hospitalisation from influenza. During pregnancy, women experience physiological changes in their cardiopulmonary and immunological systems.1, 2 An increase in oxygen consumption, a decrease in lung capacity and the suppression of cell‐mediated immunity to tolerate the growth of a genetically foreign foetus all increase pregnant women's susceptibility to infectious diseases and respiratory pathogens such as influenza.3, 4, 5 The risks of hospitalisation and complications for respiratory illness during the influenza season are higher for pregnant women and increase by trimester.6, 7 Furthermore, pregnant women infected with influenza might be more likely to have adverse birth outcomes.3, 8, 9, 10
Vaccination is the most effective preventative measure against influenza infection,8, 11 and influenza vaccines have been recommended for use in pregnant women for many decades.12 The safety, effectiveness and immunogenicity of influenza virus vaccines during pregnancy have been studied extensively, and there is good evidence to support current vaccination recommendations.13, 14, 15, 16 The World Health Organization and the US Centers for Disease Control and Prevention prioritise pregnant women for vaccination,17 and the Advisory Committee on Immunization Practices and the American College of Obstetricians and Gynecologists have recommended the inactivated seasonal influenza vaccine to women in any trimester since 2004.17, 18 Evidence of additional benefits of maternal influenza vaccination, such as the protection of young infants via placental transfer of protective antibodies to the foetus, provides further support for antenatal vaccination.19, 20 Moreover, the interruption of influenza virus transmission by vaccinating the mother, together with transplacental transfer of vaccine‐associated antibody, also reduces the risk of infection for infants 3‐4 months old (before direct vaccination is possible).21
Despite the heightened risk of influenza illness in pregnant women and benefits of vaccination, vaccination coverage rates in this population remain suboptimal. In recent years, coverage rates in the United States and Australia have ranged from 20%‐50%.22, 23, 24, 25 Surveys have attributed these low vaccine uptake rates in part to distrust in the healthcare system, unawareness of the risks of influenza infection during pregnancy, concerns about vaccine safety for the foetus and lack of encouragement from healthcare professionals.8, 22, 23, 24
Recommendations for the timing of influenza vaccination during pregnancy have varied. Although immunisation is now recommended for women at any stage of pregnancy,26 the timing of vaccination to optimise benefit to the mother and their infants is not well established. A structured analysis of the optimal timing of influenza vaccination during pregnancy would inform specific scheduling recommendations to pregnant women and maximise the benefit received by vaccination.
Previous reviews of antenatal influenza vaccination have reported limited and mixed evidence on the association between influenza vaccination, influenza infection and adverse birth outcomes, and have not examined the relationship between vaccination timing and immunogenicity.21, 27, 28, 29, 30, 31, 32 This systematic review examined whether the timing of influenza vaccination during pregnancy affects the immunogenicity of the vaccine in the mother and transplacental transfer of antibody to the newborn.
2. METHODS
This review was conducted in accordance with the PRISMA33 checklist (Table S1).
2.1. Eligibility criteria
Our population of interest was women vaccinated during pregnancy with a seasonal or pandemic vaccine. Each study was required to include women vaccinated in different trimesters to enable comparison of outcomes between trimesters. All study designs were eligible for inclusion.
The primary outcome was geometric mean titre (GMT) measured by haemagglutination inhibition (HI) assays. Geometric mean titres had to be measured either at (a) both pre‐vaccination and within one month post‐vaccination, (b) both post‐vaccination and delivery in the mother, or (c) delivery in cord blood or newborn blood (hereafter referred to as cord blood for simplicity). If reported in the included studies, seroprotection (HI titre of ≥1:40) and seroconversion (≥4‐fold increase in HI titre) rates were also discussed.34 We did not assess vaccine safety or adverse outcomes in the mother or newborn.
2.2. Search strategy
A systematic search of PubMed, Scopus, Web of Science and EMBASE was conducted across records published in English from inception to June 2016 using the following search terms with no field restrictions:
[influenza OR flu OR H1N1] AND.
[vaccin* OR immuni*] AND.
[mother OR pregnan* OR maternal OR antenatal OR trimester] AND.
[season* OR pandemic OR time OR timing OR monovalent OR inactivated OR TIV OR trivalent OR IIV].
The records identified were assessed for eligibility in three phases by two independent reviewers (WC and RM) (Figure 1): screening by title, abstract and full‐text review. A third reviewer (NG) resolved any inconsistencies. Reference lists of papers that were identified through the database search were also searched for additional studies. If the study had not stratified GMTs by trimester of vaccination, authors were contacted to provide the required data. Grey literature was not searched.
Figure 1.
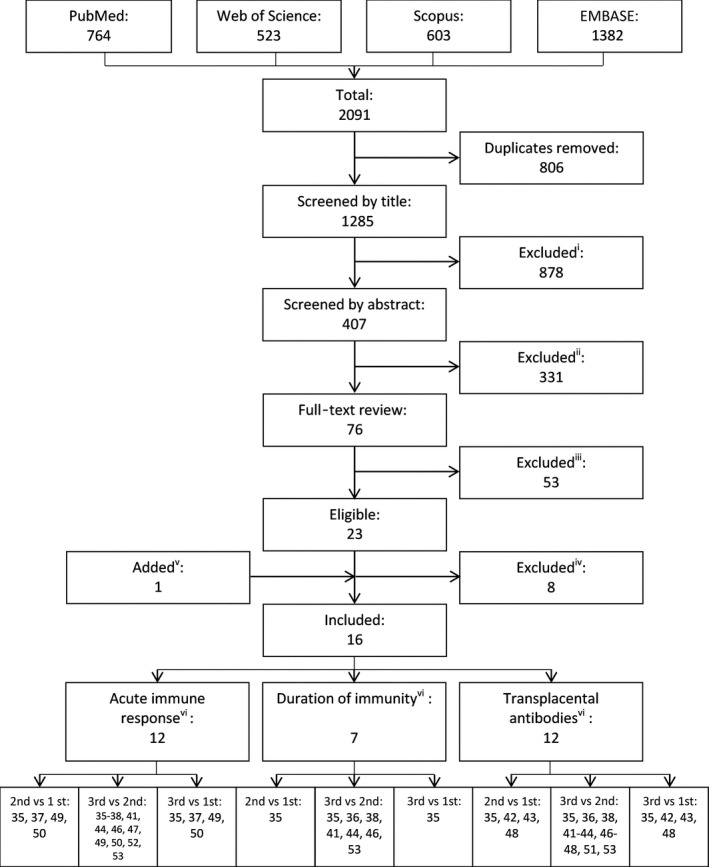
Flow diagram detailing the study inclusion/exclusion process. Reasons for inclusion/exclusion: (i) Article was a review, recommendation, statement, did not study pregnant women, did not report on immunogenicity, or did not study influenza vaccine. (ii) Article was a cost‐benefit analysis, studied vaccination uptake or attitudes, did not study pregnant women, did not report on immunogenicity, or did not study influenza vaccine. (iii) Article (n = 1) was a review of an included study, evaluated only adverse birth outcomes (n = 25), did not include any data on vaccination timing (n = 24), or only studied women in one trimester (n = 3). (iv) 19 studies contacted for stratified data: no response (n = 5), data not eligible due to time‐points measured (n = 1), declined (n = 1), data published in another included study (n = 1). (v) One article identified through hand‐searching of reference lists. (vi) References of studies included in each analysis
2.3. Data extraction
Summary characteristics of each study (study period, design, sample size, location and population, trimester of vaccination and vaccine administered) and outcome measures (GMTs, standard deviation, confidence intervals, seroprotection and seroconversion rates) were extracted and tabulated by one reviewer (WC) (Table 1 and Table S2). The methodological validity and internal bias of all included studies were assessed using critical appraisal tools ROBINS‐I for non‐randomised trials (including single‐arm studies35, 36, 37, 38) and RoB 2.0 for randomised trials (Table S3).
Table 1.
Summary characteristics of included studies
| Study [ref] (year; setting) | Study design | Vaccine (season)a | Sample size with available data at each time‐pointb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐vaccination | Post‐vaccinationc | Mother at delivery | Cord bloodd | |||||||||||
| 1st TRI | 2nd TRI | 3rd TRI | 1st TRI | 2nd TRI | 3rd TRI | 1st TRI | 2nd TRI | 3rd TRI | 1st TRI | 2nd TRI | 3rd TRI | |||
| Seasonal epidemics | ||||||||||||||
| Kostinov et al47 (2015; MOW, RUS) | Prospective cohort | TIV Agrippal S1 (2 seasons: 2010/11, 2012/13) | – | 27 | 21 | – | 27 | 21 | – | – | – | – | 23 | 19 |
| Madhi et al44 (2014; SOW, RSA) | Randomised controlled trial | TIV Vaxigrip (2 seasons: 2011 & 2012) | – | 59 | 83 | – | 59 | 83 | – | 59 | 83 | – | 35 | 58 |
| Blanchard‐Rohner et al52 (2013; GVA, SUI) | Cross‐sectional | TIV Mutagrip (2010/11) | – | – | – | – | – | – | – | – | – | 4 | H1N1: 38; H3N2: 39; B: 38 | H1N1: 56; H3N2: 57; B:56 |
| Christian et al48 (2013; OH, USA) | Prospective cohort | TIV Fluarix (2011/12) | 4 | 15 | 8 | 4 | 15 | 8 | – | – | – | – | – | – |
| Garcia‐Putnam et al46 (2013; NC, USA) | Prospective cohort | TIV Fluarix (2011/12) | – | 12 | 16 | – | 12 | 16 | – | 12 | 16 | – | 12 | 16 |
| Lin et al36 (2013; TPE, TWN) | Prospective cohort | TIV AdimFlu‐S (2011/12) | – | 16 | 30 | – | 16 | 27 | – | 15 | 29 | – | 15 | 27 |
| Schlaudecker et al49 (2012; OH, USA) | Prospective cohort | TIV Fluarix (2011/12) | 4 | 19 | 6 | 4 | 19 | 6 | – | – | – | – | – | – |
| Eick et al53 (2011; USA) | Prospective cohort | TIVs (3 seasons: 2002/03, 2003/04, 2004/05) | – | – | – | – | – | – | – | – | – | – | H1N1: 123; H3N2: 60/60; B: 92/63 | H1N1: 390; H3N2: 267/267; B:192/123 |
| Yamaguchi et al38 (2009; TYO, JPN) | Prospective cohort | TIV FLUBIK HA (2007/08) | – | 53 | 72 | – | 39 | 71 | – | 53 | 70 | – | 54 | 73 |
| 2009 pandemic | ||||||||||||||
| Bischoff et al50 (2013; CPH, DEN) | Randomised controlled trial | U/A and MF59‐adj Focetria (A/H1N1/pdm09) | – | 15 µg U/A: 41; 7.5 µg F/A: 31; 3.75 µg H/A: 11 | 15 µg U/A: 18; 7.5 µg F/A: 21; 3.75 µg H/A: 18 | – | 15 µg U/A: 41; 7.5 µg F/A: 31; 3.75 µg H/A: 11 | 15 µg U/A: 18; 7.5 µg F/A: 20; 3.75 µg H/A: 18 | – | – | – | – | – | – |
| Chao et al43 (2013; TWN) | Prospective cohort | U/A and MF59‐adj AdimFlu‐S (A/H1N1/pdm09) | – | – | – | – | – | – | – | – | – | U/A: 8; Adj: 7 | U/A: 15; Adj: 2 | U/A: 8; Adj: 1 |
| Fisher et al42 (2012; CO, USA) | Prospective cohort | Monovalent vaccine (A/H1N1/pdm09) | – | – | – | – | – | – | – | – | – | 7 | 3 | 4 |
| Horiya et al35 (2011; TYO, JPN) | Prospective cohort | Monovalent vaccine (A/H1N1/pdm09) | 1D:17; 2D:17 | 1 D: 48; 2 D: 79 | 1 D: 35; 2 D: 29 | 1D: –; 2D:17/17 | 1 D: –; 2 D: 78/79 | 1 D: –; 2 D: 29/28 | 1 D: –; 2 D: 16 | 1 D: –; 2 D: 77 | 1 D: –; 2 D: 28 | 1D: 1; 2D: 16 | 1 D: 48; 2 D: 77 | 1 D: 35; 2 D: 28 |
| Jackson et al41 (2011; SEA, USA) | Randomised controlled trial | Monovalent vaccine (A/H1N1/pdm09) | – | 25 µg: 32; 49 µg: 42 | 25 µg: 23; 49 µg: 16 | – | 25 µg: 32/24; 49 µg: 42/37 | 25 µg: 23/14; 49 µg: 16/14 | – | 25 µg:26; 49 µg: 40 | 25 µg:15; 49 µg: 7 | – | 25 µg:25; 49 µg: 39 | 25 µg: 14; 49 µg: 7 |
| Ohfuji et al37 (2011; OSA, JPN) | Prospective cohort | Monovalent vaccine (A/H1N1/pdm09) | 26 | 46 | 77 | 26/26 | 46/46 | 77/77 | – | – | – | – | – | – |
| Tsatsaris et al51 (2011; FRA) | Prospective cohort | Monovalent vaccine (A/H1N1/pdm09) | – | 58 | 49 | – | 55 | 46 | – | 52 | 47 | – | 47 | 41 |
TIV, trivalent influenza vaccine; Kostinov et al & Madhi et al used same vaccine in both seasons, respectively.
TRI, trimester (1st TRI: <14 wk, 2nd TRI: ≥14 wk/<28 wk, 3rd TRI: ≥28 wk; where not otherwise specified, sample sizes are identical in analyses of each strain (H1N1, H3N2, B); Madhi et al participants were HIV‐negative; Eick et al display values for two H3N2 and two B strains, separated by a slash (2002/’03 & 2003/’04 vaccines contained the same strains, 2004/’05 contained different H3N2 & B strains); U/A: unadjuvanted, F/A: full‐adjuvanted, H/A: half‐adjuvanted; Adj: adjuvanted; D: dose; Horiya et al, Jackson et al and Ohfuji et al display post‐vaccination titres after 1st and 2nd dose, separated by a slash; “‐”: HI titres not measured at this time‐point.
1 month post‐vaccination: Kostinov et al, Madhi et al, Christian et al, Yamaguchi et al; 4 wk post‐vaccination: Lin et al, Garcia‐Putnam et al, Schlaudecker et al; 3 wk after vaccination: Bischoff et al, Horiya et al, Jackson et al, Ohfuji et al, Tsatsaris et al; 3 wk post‐2nd dose: Horiya et al & Jackson et al; 4 wk post‐2nd dose: Ohfuji et al
Kostinov et al blood taken from newborn 2‐3 d post‐delivery, Madhi et al blood taken from newborn ≤ 7 d post‐delivery, Eick et al blood taken from newborn ≤ 14 d post‐delivery if cord blood was unavailable.
2.4. Analysis
Three analyses of study data were conducted. First, the effect of vaccination trimester on acute immune response was measured by calculating a ratio of GMT fold increases (GMFI) comparing women vaccinated in different trimesters; this ratio used a pre‐vaccination GMT and a post‐vaccination GMT (either three weeks, four weeks, or one month after vaccination). Second, the effect of vaccination trimester on antibody persistence was measured by calculating a ratio of GMT fold decreases (GMFD) comparing women vaccinated in different trimesters; this ratio used a post‐vaccination GMT (measured either three weeks, four weeks, or one month after vaccination) and a maternal GMT measured at the time of delivery. Third, the effect of vaccination trimester on transplacental antibodies was measured by calculating a ratio of cord blood GMTs (GMR) comparing women vaccinated in different trimesters; this ratio used only the cord blood time‐point.
Ratios of GMTs were used to account for anticipated differences in baseline seropositivity between seasons. Relative change in GMT is an appropriate measure to account for this heterogeneity, allowing the comparison of women vaccinated with different vaccines, undergoing different protocols and with different demographic characteristics.
Where not otherwise reported, standard errors for GMTs at each time‐point were calculated from reported 95% confidence intervals (CI) using the t‐distribution or from standard deviations. Standard errors of ratios were calculated using the z‐distribution or, if the sample size did not change between time‐points, the t‐distribution. All analyses were conducted on the logarithmic scale and back‐transformed to the original scale. Heterogeneity was examined using forest plots and the I2 statistic. Due to the small number of studies included, funnel plots to check for publication bias were not produced.39 Data were summarised using a random‐effect (DerSimonian and Laird) model to accommodate between‐study heterogeneity in true effects, and the estimate and 95% CI of the effect were presented on the logarithmic scale.40
Timing of vaccination was stratified by trimester (1st trimester: <14 weeks, 2nd trimester: 14‐27 weeks, 3rd trimester: ≥28 weeks). Accordingly, three comparisons were made: 2nd‐trimester vaccination compared with 1st‐trimester vaccination, the 3rd trimester compared with the 2nd and the 3rd trimester compared with the 1st. For studies that included a two‐dose vaccine group, these data were analysed in separate meta‐analyses (trimester of vaccination defined by the timing of the first dose).35, 37, 41 Seven primary meta‐analyses (Figure 2, 3, 4 & Figures S1, S7, S12, S16) were conducted, plus four separate meta‐analyses of results after a second dose (Figures S2, S3, S8, S9). Within each meta‐analysis, results were presented across all virus strains and by virus strain. We did not adjust for multiple testing.
Figure 2.
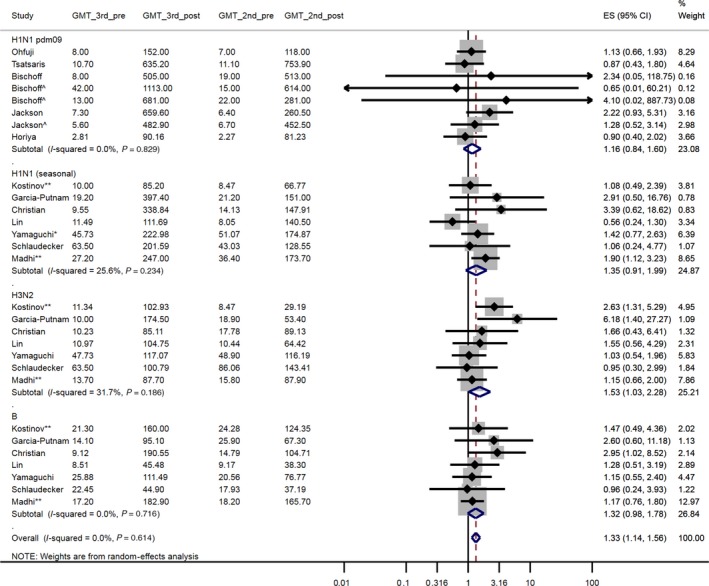
A forest plot of the geometric mean fold increase (GMFI) ratio, pre‐vaccination to post‐vaccination, comparing women vaccinated in the 3rd trimester with women vaccinated in the 2nd trimester. ^ Bischoff et al 7.5 µg & 3.75 µg groups, respectively (as opposed to the 15 µg group); Jackson et al 49 µg group (as opposed to the 25 µg group). * Yamaguchi et al studied a non‐pdm09 H1N1 strain (2007/08 season). ** Kostinov et al & Madhi et al was conducted over two influenza seasons (using same vaccine in both seasons). GMT_3rd_pre: geometric mean titre (GMT) pre‐vaccination, 3rd trimester vaccination. GMT_3rd_post: GMT post‐vaccination, 3rd trimester vaccination. GMT_2nd_pre: GMT pre‐vaccination, 2nd trimester vaccination. GMT_2nd_post: GMT post‐vaccination, 2nd trimester vaccination. ES (95% CI): Effect size (GMFI) (95% confidence interval)
Figure 3.
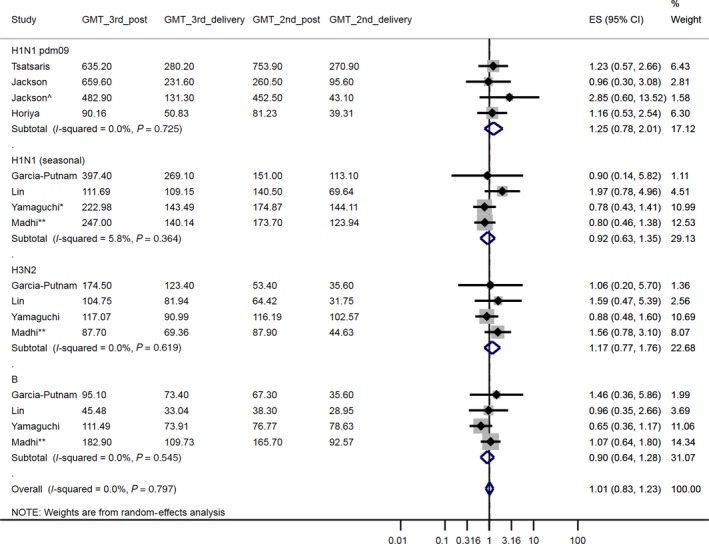
A forest plot of the geometric mean fold decrease (GMFD) ratio, post‐immunisation to delivery, comparing women vaccinated in the 3rd trimester with women vaccinated in the 2nd trimester. ^ Jackson et al 49 µg group (as opposed to the 25 µg group). * Yamaguchi et al studied a non‐pdm09 H1N1 strain (2007/08 season). ** Madhi et al was conducted over two influenza seasons (using same vaccine in both seasons). GMT_3rd_ post: geometric mean titre (GMT) post‐vaccination, 3rd trimester vaccination. GMT_3rd_delivery: GMT in mother at delivery, 3rd trimester vaccination. GMT_2nd_ post: GMT post‐vaccination, 2nd trimester vaccination. GMT_2nd_delivery: GMT in mother at delivery, 2nd trimester vaccination. ES (95% CI): Effect size (GMFD) (95% confidence interval)
Figure 4.
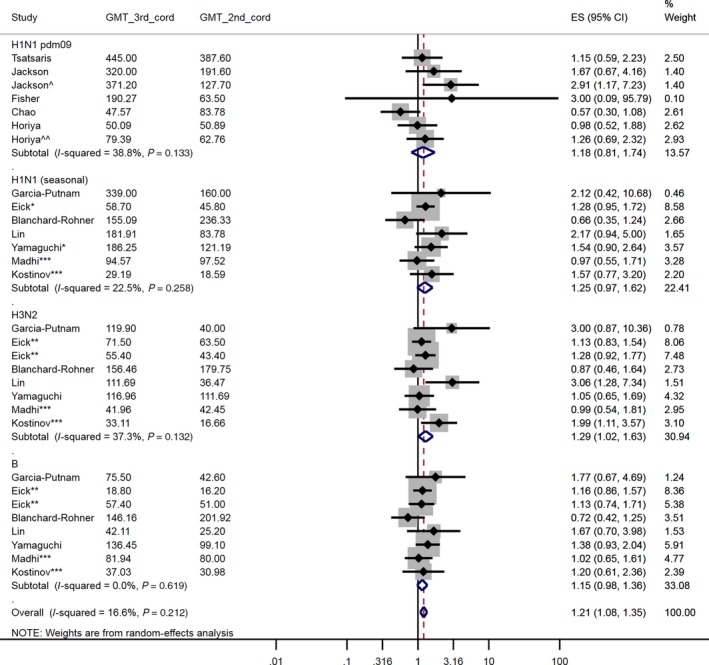
A forest plot of the cord‐blood GMT ratio comparing women vaccinated in the 3rd trimester with women vaccinated in the 2nd trimester. ^ Jackson et al 45 µg dose group (as opposed to the 25 µg dose group) – all women had received two vaccine doses. ^^ Horiya et al two vaccine dose group (as opposed to the one vaccine dose group). * Eick et al & Yamaguchi et al studied non‐pdm09 H1N1 strain (2002‐05 seasons and 2007/08 season, respectively). ** Eick et al contained different H3N2 and two B strains in two of the three seasons (‘02/’03 & ‘03/’04 vaccines contained the same strains, ‘04/’05 contained different H3N2 & B strains). *** Madhi et al and Kostinov et al were conducted over two influenza seasons (using same vaccine in both seasons). GMT_3rd_cord: geometric mean titre (GMT) in cord‐blood at delivery, 3rd trimester vaccination. GMT_2nd_cord: GMT in cord‐blood at delivery, 2nd trimester vaccination. ES (95% CI): Effect size (GMR) (95% confidence interval)
We also examined seroprotection and seroconversion rates reported in the included studies (Table S2). Where possible, we explored these outcomes stratified by trimester of vaccination; however, a limited number of studies reported these stratified data. We included some statistical results from the original papers (eg p‐values from comparisons of seroprotection and seroconversion proportions).
For the acute immune response and transplacental antibody outcomes, we conducted three sensitivity analyses for the 3rd‐ versus 2nd‐trimester comparison in which we restricted the studies included in the meta‐analysis to those that also vaccinated women in the 1st trimester (Figures S4, S5, S15). This sensitivity analysis allows us to explore whether the findings based on studies that included women vaccinated in the 1st trimester (as well as the 2nd and 3rd) may have differed from those of studies that included only women vaccinated in the 2nd and 3rd trimesters due to participant recruitment. This analysis was not performed for the antibody persistence outcome because there was only one study that included women vaccinated in the 1st trimester.35 Six other sensitivity analyses were undertaken whereby studies with concerns of bias (due to small sample size42, 43 or unbalanced loss to follow‐up between study groups38, 41, 44) were excluded (Table S3).
All analyses included all available cases with the unit of analysis being the participant. Data preparation and manipulation were undertaken using Microsoft Excel, and all meta‐analyses and forest plots were produced in Stata 14.2.45
3. RESULTS
3.1. Literature search
After duplicates were removed, 1,285 articles were identified from the electronic databases search (Figure 1). 878 and 331 articles were excluded after screening by title and abstract, respectively. Full‐text assessment of the remaining 76 studies left 23 eligible studies. One additional study was identified from the reference lists of the included studies. Of these 24 studies, five had complete data. We contacted authors of the other 19 studies, 11 of whom provided additional data.
In total, 16 studies were included. Nine were conducted during seasonal epidemics (two prior to the 2009 pandemic) and seven during the H1N1/pdm09 year; 12 were cohort studies, three were randomised controlled trials (RCTs), and one was a cross‐sectional study (Table 1). One was a conference abstract.46 Spanning a decade (2002‐2012), this systematic review includes studies conducted in eight different countries and during eight different influenza seasons.
3.2. Acute immune response
Twelve studies measured both baseline HI titres immediately prior to vaccination and post‐vaccination HI titres (Table S2). Post‐vaccination blood draw occurred at 1 month after vaccination in four studies,38, 44, 47, 48 at 4 weeks in three studies36, 46, 49 and at 3 weeks in five studies.35, 37, 41, 50, 51 Horiya et al35 and Jackson et al41 also measured HI titres 3 weeks after a second dose, whereas Ohfuji et al37 measured titres at 4 weeks after the second dose (compared to 3 weeks after the first dose). These pre‐ and post‐vaccination time‐points allowed us to assess the production of antibodies induced by vaccination (geometric mean titre fold increase (GMFI)). For this outcome, four studies35, 37, 48, 49 included women vaccinated in any trimester while the remaining eight studies included only women vaccinated in the 2nd and 3rd trimester.
3.2.1. 2nd versus 1st trimester
Across all influenza strains, the GMFI for women vaccinated in the 2nd trimester was 1.45 [95% CI: 0.98, 2.14] times greater than those vaccinated in the 1st trimester (Figure S1; 4 studies, 210 participants). Subgroup analyses within each influenza subtype also revealed effect sizes in this direction. Only Horiya et al35 had a lower GMFI in the 2nd trimester (after 1st dose: 0.87 [0.33, 2.26]).
After a second dose, Horiya et al35 and Ohfuji et al37 both showed a greater GMFI for women vaccinated in the 2nd trimester than 1st trimester (1.16 [0.44, 3.08] and 1.16 [0.59, 2.29], respectively) (Figure S2; 2 studies, 168 participants).
3.2.2. 3rd versus 2nd trimester
The GMFI for women vaccinated in the 3rd trimester was 1.33 [95% CI: 1.14, 1.56] times greater than those vaccinated in the 2nd trimester (Figure 2; 12 studies, 1028 participants). Studies by Kostinov et al47 and Madhi et al44 were conducted over two influenza seasons and both had robust results for at least one subtype (H3N2 for Kostinov et al47:2.63 [1.31, 5.29] and H1N1 for Madhi et al44:1.90 [1.12, 3.23]).
After a second dose, the GMFI for women vaccinated in the 3rd trimester was 1.34 [0.93, 1.93] times greater than for women vaccinated in the 2nd trimester (Figure S3; 3 studies, 344 participants). However, this trend was not consistent across all three studies.
Restricting the meta‐analysis to only studies that also vaccinated women in the 1st trimester35, 37, 48, 49 slightly lowered the pooled GMFI from 1.33 [1.14, 1.56] to 1.25 [0.89, 1.76] (Figures S4 and S5; 4 studies/279 participants and 2 studies/231 participants, respectively). In another sensitivity analysis, excluding Yamaguchi et al38 due to risk of internal bias, the pooled GMFI was 1.36 [1.15, 1.61] (Figure S6; 11 studies, 903 participants).
3.2.3. 3rd versus 1st trimester
The clarity of a dose‐response relationship by trimester is strengthened by comparing women vaccinated in the 3rd and 1st trimester: the GMFI for women vaccinated in the 3rd trimester was 1.96 [95% CI: 1.01, 3.82] times greater than those vaccinated in the 1st trimester (Figure S7; 4 studies, 171 participants). There was also a similar trend after a second dose (1.56 [0.89, 2.74]; Figure S8; 2 studies, 149 participants).
3.3. Antibody persistence
In addition to the post‐vaccination blood draw, seven studies35, 36, 38, 41, 44, 46, 51 also measured HI titres in the mother at delivery (Table S2). Thus, we can quantify the reduction in antibodies between the post‐vaccination immune response and delivery (geometric mean titre fold decrease (GMFD)). All seven studies included women vaccinated in the 2nd and 3rd trimester, while only one35 included women in all three trimesters.
3.3.1. 2nd versus 1st trimester
Horiya et al35 were the only study to include women vaccinated in all three trimesters; therefore, no meta‐analysis was possible. In this study, the fold reduction in GMT was greater for women vaccinated in the 1st trimester compared with women vaccinated in the 2nd trimester (1.59 [95% CI: 0.56, 4.54]). This difference was smaller when comparing women who received two vaccine doses during their pregnancy (1.19 [0.41, 3.49]).
3.3.2. 3rd versus 2nd trimester
The fold reduction in GMT from immunisation to delivery was similar for women vaccinated in the 2nd and 3rd trimesters (1.01 [95% CI: 0.83, 1.23]) (Figure 3; 7 studies, 658 participants). A notable exception was the study by Yamaguchi et al,38 in which the GMFD was smaller following 2nd trimester immunisation across all strains (H1N1: 0.78 [0.43, 1.41], H3N2: 0.88 [0.48, 1.60], B: 0.65 [0.36, 1.17]).
After a second dose, the GMFD between immunisation and delivery for women vaccinated in the 3rd trimester was less than for women vaccinated in the 2nd trimester (1.28 [0.70, 2.35] times; Figure S9; 2 studies, 202 participants).
A sensitivity analysis in which Madhi et al44 and the 49 µg dose group of Jackson et al41 were excluded due to risk of internal bias (Table S3) had a minor impact on the effect size (after 1 dose: 0.97 [0.76, 1.24]; after 2 doses: 1.10 [0.57, 2.10]; Figures S10 and S11; 7 studies/600 participants and 2 studies/148 participants, respectively).
3.3.3. 3rd versus 1st trimester
Comparable to the 2nd‐ versus 1st‐trimester analysis of the same study, Horiya et al35 demonstrated that the fold reduction in GMT was greater for women vaccinated in the 1st trimester compared with women vaccinated in the 3rd trimester (1.86 [95% CI: 0.60, 5.76]). This difference was smaller when comparing women who received two vaccine doses during their pregnancy (1.28 [0.40, 4.05]).
3.4. Transplacental antibodies
Twelve studies measured HI titres in cord blood at delivery (Table S2). Four studies35, 42, 43, 52 included women vaccinated in any trimester while eight studies included only women vaccinated in the 2nd and 3rd trimester. This time‐point enables the direct comparison of antibodies transferred to the foetus by trimester of vaccination (geometric mean titre ratio (GMR)).
3.4.1. 2nd versus 1st trimester
Women vaccinated in the 2nd trimester had a higher cord blood GMT (1.64 [95% CI: 1.21, 2.24]) than those immunised earlier in pregnancy (Figure S12; 4 studies, 178 participants). All point estimates were greater than one, and the pooled effect size in the subgroup analysis of the H1N1pdm09 strain was robust (1.50 [1.03, 2.19]). All women in Horiya et al35 received two vaccine doses, and the cord blood GMR by trimester in this study was similar to other studies that administered only one dose (1.59 [0.75, 3.38]). In Chao et al,43 the cord blood GMTs post‐1st and post‐2nd trimester vaccination with adjuvanted vaccine were less different than after vaccination with unadjuvanted vaccine (unadjuvanted GMR: 1.62 [0.89, 2.94]; adjuvanted GMR: 1.22 [0.62, 2.39]) although confidence intervals of both ratios included 1.
A sensitivity analysis in which results from Fisher et al42 and the adjuvanted vaccine group in Chao et al43 were excluded due to risk of internal bias (Table S3) had minimal impact on the pooled effect size (1.76 [1.23, 2.51], Figure S13; 3 studies, 159 participants).
3.4.2. 3rd versus 2nd trimester
Consistent with the 2nd‐ versus 1st‐trimester comparison above, women vaccinated in the 3rd trimester had a 1.21 [95% CI: 1.08, 1.35] times higher cord blood GMT than women vaccinated in the 2nd trimester (Figure 4; 12 studies, 1332 participants). The results from Horiya et al35 and Jackson et al41 suggest that this relationship between vaccination in the later trimester and higher cord blood titres is accentuated with a second dose (3 weeks after the first) or with a higher dose. Eick et al53 and Yamaguchi et al38 studied different seasonal H1N1 strains prior to 2009; however, their GMT cord blood ratios by trimester are similar to studies of H1N1pdm09 vaccines. Furthermore, Eick et al53 took place over three influenza seasons with three vaccines comprising two unique H3N2 and B strains (the H1N1 strain was the same in all three vaccines); the cord blood GMTs for both H3N2 and B strains between women vaccinated in the 3rd and 2nd trimesters were virtually equivalent.
A sensitivity analysis in which results from Fisher et al42 and the 45 µg dose group in Jackson et al41 were excluded due to risk of internal bias (Table S3) had minimal impact on the pooled effect size (1.19 [1.07, 1.33], Figure S14; 11 studies, 1279 participants).
Restricting the meta‐analysis to only studies that also vaccinated women in the 1st trimester35, 42, 43, 52 changed the direction of the pooled effect (0.82 [0.64, 1.06], (Figure S15; 4 studies, 314 participants)). However, this result is strongly influenced by a reduction in studies with GMT cord blood ratios greater than one.
3.4.3. 3rd versus 1st trimester
Women vaccinated in the 3rd trimester had a cord blood GMT 1.44 [95% CI: 0.95, 2.19] times higher than those immunised in the 1st trimester (Figure S16; 4 studies, 131 participants). Even though Horiya et al35 include only women who received two vaccine doses, the cord blood GMR by trimester in this study was similar to other single‐dose studies (2.01 [0.84, 4.82]). Fisher et al42 had a much larger effect size but were also far less precise (8.62 [0.38, 194.16]).
A sensitivity analysis in which Fisher et al42 were excluded due to risk of internal bias (Table S3) had minimal impact on the pooled effect size (1.40 [0.93, 2.10], Figure S17; 3 studies, 121 participants).
3.5. Seroprotection and seroconversion
3.5.1. Acute immune response
With the exception of women vaccinated in the 2nd trimester in Kostinov et al,47 seroprotection and seroconversion rates were consistently in excess of 75% irrespective of the timing of vaccination. Compared with women vaccinated in the 2nd trimester, Kostinov et al47 reported that women vaccinated in the 3rd trimester had a significantly higher seroconversion rate (P < 0.01) against the H3N2 subtype (3rd trimester: 73%; 2nd trimester: 30%) and influenza B (3rd trimester: 82%; 2nd trimester: 52%). Ohfuji et al37 only found a significant difference in seroconversion rates by trimester four weeks after administering a second vaccine dose (1st trimester: 76%, 2nd trimester: 91%, 3rd trimester: 94% (P = 0.02)). Similarly, Horiya et al35 found a non‐significant increase in HI titres after a second dose; there were no differences by trimester of vaccination (90% seroconversion rate regardless of timing). In addition, neither Tsatsaris et al51 nor Garcia‐Putnam et al46 found any significant differences in seroconversion or seroprotection rates by trimester, although Garcia‐Putnam et al46 reported a similar trend with a seroprotection rate of 100% against the H1N1 subtype for women vaccinated in the 3rd trimester and 75% for those vaccinated in the 2nd trimester.
The remaining studies did not stratify seroprotection and seroconversion rates by trimester at this time‐point36, 38, 41, 48, 49, 50; generally, seroprotection rates were very high (H1N1: 88‐100%; H3N2: 81‐100%; B: 57‐83%), while seroconversion rates were low (H1N1: 51‐97%; H3N2: 10‐63%; B: 21‐63%) (see Limitations).
3.5.2. Antibody persistence
Compared with women vaccinated in the 2nd trimester, Kostinov et al47 demonstrated a greater seroprotection rate for 3rd trimester vaccinated women in the days following delivery (P < 0.01). Furthermore, comparing HI titres one month after vaccination and at delivery, Yamaguchi et al38 found women vaccinated in the 3rd trimester maintained elevated antibody levels (>1:40) more effectively than did women vaccinated in the 2nd trimester (2nd trimester: 66% ± 27% (standard deviation); 3rd trimester: 94% ± 31% (P < 0.001)). In contrast, Tsatsaris et al51 reported similar seroprotection rates at delivery for both women vaccinated in the 2nd and 3rd trimester (2nd trimester: 92% [95% CI: 81%, 98%]; 3rd trimester: 91% [80%, 98%]). Protection extending beyond delivery was also measured by Kostinov et al,47 who found no difference in seroprotection rates for women vaccinated in different trimesters at three or six months after delivery; however, both groups had experienced a significant decrease in seroprotection.
Further evidence of waning protection was reported by Lin et al36 and Fisher et al,42 who both found a significant decrease in antibody titres between vaccination and delivery, with Lin et al36 using linear regression to estimate that HI titres fall below 1:40 after 150 days. Horiya et al35 reported a non‐significant association between higher HI titres at delivery and vaccination in later stages of pregnancy, and for women who received two vaccine doses. Jackson et al41 also established that a longer vaccination‐to‐delivery interval resulted in lower HI titres at delivery (P < 0.05), however did not find a difference in seroprotection rates in the mother at delivery between 25 µg and 49 µg dose groups (25 µg: 85% [71%, 94%]; 49 µg: 62% [46%, 75%]). Finally, all three vaccine groups in Bischoff et al50 maintained high seroprotection and seroconversion rates for at least three months after vaccination, and only the 7.5 µg full‐adjuvanted group showed a significant decrease in protection after 10 months (3 months: seroconversion rate: 95% [82%, 99%], seroprotection rate: 96% [88%, 100%]; 10 months: seroconversion rate: 59% [39%, 76%], seroprotection rate: 70% [55%, 83%]).
Even though there was some evidence of higher antibody levels at delivery in women vaccinated later in pregnancy, seroprotection and seroconversion rates at delivery were generally high regardless of vaccination timing.36, 41, 42, 43, 46
3.5.3. Transplacental antibodies
Kostinov et al47 found that infants born to women vaccinated in the 3rd trimester had significantly higher seroprotection rates 2‐3 days after delivery (P < 0.01), compared with infants born to women vaccinated in the 2nd trimester (2nd trimester: H1N1: 39%, H3N2: 37%; 3rd trimester: H1N1: 68%, H3N2: 76%). Both groups of infants had decreased seroprotection rates three months later; however, rates remained higher (P < 0.01) for the 3rd trimester group (2nd trimester: H1N1: 16%, H3N2: 11%, B: 26%; 3rd trimester: H1N1: 26%, H3N2: 37%, B: 42%). In contrast, neither Tsatsaris et al51 nor Eick et al53 found any significant difference in cord blood seroprotection rates between women vaccinated in the 2nd or 3rd trimesters. Furthermore, Yamaguchi et al38 reported that the ratio between HI titre in maternal blood at delivery and cord blood was higher for women vaccinated in the 2nd trimester (2nd trimester: 161%; 3rd trimester: 127% (P = 0.02)). Finally, compared with no vaccination, Blanchard‐Rohner et al52 found vaccination less than 15 days before delivery did not result in a significant increase in seroprotection rate at delivery.
The remaining studies did not stratify seroprotection and seroconversion rates by trimester at this time‐point.35, 36, 41, 43, 44 While Jackson et al41 found no difference in cord blood seroprotection between 25 µg and 49 µg dose groups, Horiya et al35 found non‐significantly higher rates for women vaccinated in later stages of pregnancy and for women who received two vaccine doses.
3.6. Risk of bias
Largely influenced by the nature of the intervention and the outcome measures, we determined that there was low risk of internal methodological bias in all included studies (Table S3). However, the main limitations were low numbers of participants, missing data or loss to follow‐up (Table 1). In Jackson et al,41 56% of participants in the 49 µg dose group were missing data at delivery, while in Madhi et al,44 36% of women vaccinated in the 2nd trimester were missing data at delivery (using per‐protocol participants). Similarly, 26% of those vaccinated in the 2nd trimester in Yamaguchi et al38 were missing data at 28 days post‐vaccination. Those vaccinated with an adjuvanted vaccine in Chao et al43 (ten in total) included only two participants in the 2nd trimester and only one in the third trimester (not included in the analysis). Fisher et al42 also had a very small sample size (1st trimester: 7, 2nd trimester: 3, 3rd trimester: 4). The effects of these studies on the pooled effect sizes were explored through sensitivity analyses (Figures S6, S10, S11, S13, S14, S17).
4. DISCUSSION
4.1. Summary of findings
This systematic review explored the association between the timing of an influenza vaccination given during pregnancy and immunogenicity in the mother and newborn. The three main findings of this review were as follows. First, women vaccinated later during pregnancy had a greater immune response to vaccination. This effect size increased from the 1st to 3rd trimester. Second, maternal antibodies at delivery were reduced by a similar factor relative to post‐immunisation titres, regardless of whether women were vaccinated in the 2nd or 3rd trimester. This observation suggests that antibodies wane faster in women vaccinated later in pregnancy; however, this hypothesis could not be explored further due to the low number of studies that vaccinated women in the 1st trimester. Regardless, antibody waning seems to occur over a fairly short time frame; some studies have estimated the antibody half‐life to be as short as seven weeks.54, 55 Third, despite the observation that GMT at delivery was consistent across trimesters, there was strong evidence that vaccination in a later trimester increased the transfer of antibodies to the foetus.
To gain further insight into the impact of vaccination timing, we reported on the public health–relevant outcomes of seroprotection and seroconversion. An HI titre of 1:40 is recognised as an immunologic correlate corresponding to a 50% reduction in the risk of contracting influenza.56 Even though some of the studies included in this review reported significantly higher seroprotection or seroconversion rates for women vaccinated in later trimesters at both the acute post‐vaccination time‐point and at delivery in the mother and cord blood, most women still achieved sufficient protection regardless of vaccination timing. Other studies not meeting this review's inclusion criteria have found no difference in seroprotection rates by vaccination trimester.12, 57, 58, 59
Some study designs included administering two vaccine doses, higher antigen doses or adjuvanted vaccines.35, 37, 41, 43, 50 By trimester, the immune response was not greatly impacted by higher doses or adjuvanted vaccines; neither was a substantial increase in GMT conferred after a second dose. However, those who received two doses or a higher dose tended to have higher levels of antibodies at delivery and also transferred more antibodies to the foetus.
4.2. Limitations
Of the original publications, only six reported the effect of vaccination timing on vaccine immunogenicity in detail with stratification by trimester. Thus, much of the data acquired for this review were not originally collected to address our objectives. Observational studies are also prone to inherent biases, and many of the included studies only had small numbers of participants. While RCTs are desirable for addressing the impacts of antenatal vaccination timing on vaccine immunogenicity, there are limitations on study design due to the ethical issues raised by delaying vaccination. Comparing the usefulness of multiple doses is a potential alternative. Furthermore, some of the included studies used vaccines not licensed for pregnant women, potentially limiting the generalisability of their results.
All studies in this review included women vaccinated in the second and third trimesters, but only seven studies included women vaccinated in the first trimester (likely influenced by the short time between pregnancy diagnosis and the end of first trimester). This limited the comparisons of vaccine immunogenicity against women vaccinated in the first trimester. The robustness of our results is likely affected by the low number of studies available for inclusion in these meta‐analyses (Figure 1). However, the I2 values were less than 44% in all our meta‐analyses, indicating low between‐study heterogeneity. Finally, we interpreted our results by considering overall trends rather than statistical significance and did not account for multiple comparisons.
Immunity prior to vaccination can both affect the immune response generated from the vaccine and mask the true immunogenicity of the vaccine. Seroprotection rates may over‐estimate immunogenicity, while seroconversion rates may result in under‐estimation if there is high baseline population protection. We used fold increases and fold decreases in GMT in an attempt to control for the pre‐vaccination immune state—a valid method when combined into a meta‐analysis.60 Furthermore, exposure to wild‐type influenza virus between vaccination and delivery may have impacted HI titres measured at delivery.
Finally, the widely accepted standard correlate of protection (ie based on challenge studies in healthy adults, that an HI titre >1:40 corresponds to a 50% reduction in the risk of contracting influenza) is not grounded in strong evidence.61, 62 While a higher HI titre is predictive of some protection, there is evidence to suggest that neuraminidase inhibition (NAI) titre is more predictive of protection and reduced infection.63 NAI titres were not measured in any of the included studies. There remains a need to standardise serological assays and better define correlates of protection, which may vary according to individual characteristics, populations, age groups and vaccine types.
5. IMPLICATIONS AND CONCLUSION
Vaccinating a woman in early pregnancy will provide protection against influenza for a greater proportion of pregnancy, but may increase the probability that this immunity will not last until delivery. Lower antibody levels at delivery may reduce transplacental antibodies and the benefit to the newborn, and loss of immunity in the mother may increase the chance of her becoming a viral source to the newborn. A similar issue arises with antenatal pertussis vaccination, where there is recent evidence that immunogenicity is higher in the second trimester compared with the third trimester, which has resulted in some countries bringing forward their recommendations for the optimal timing of pertussis immunisation in pregnancy.64 Furthermore, for women vaccinated early during pregnancy, but late in the influenza season, clinical protection may be reduced if the circulating strain of the following influenza season does not match the strain included in the previous season's vaccine, regardless of whether antibody levels remain high. Given that women immunised earlier in pregnancy show evidence of immune waning by the point of delivery, our findings support current recommendations for women immunised early in their pregnancy to receive a second dose if they are still pregnant in the following influenza season.
Due to the increased risk of adverse birth outcomes caused by maternal influenza infection, and the subsequent increased risk of lifelong chronic diseases associated with these birth outcomes, the economic burden of maternal infection is substantial.65 There is recent evidence that the risk of foetal death and other adverse birth outcomes is highest for women who become infected with seasonal influenza during their first trimester.66 That research compounds the complexity of optimising the scheduling of influenza vaccination for pregnant women and, in conjunction with our findings, highlights that the infant is at risk both in utero and after birth. The protection of the infant from adverse birth outcomes and infection in early life has not been measurable outcomes in this review. Nevertheless, this is a key aspect of antenatal vaccination policy and there is an extensive amount of documented interest in these benefits.51, 52, 67, 68, 69
While studies of vaccine effectiveness and efficacy were not included in this systematic review, the increased immune response by gestational age suggests that post‐vaccination influenza infection might be less likely for women vaccinated in later trimesters. However, preventing clinical disease depends more on the timing of vaccination relative to the seasonal influenza epidemic rather than to gestational age. For example, vaccination should not be delayed for a woman in her first trimester if the influenza season has begun and the vaccine is available.
The findings of this systematic review are informative and relevant to current vaccine scheduling for pregnant women. We found that women vaccinated later in pregnancy had a stronger immune response and transferred more antibodies to the foetus.
A limited number of published studies have considered the impact of vaccine timing on maternal and infant protection. We need to understand the full implications of vaccination timing for protection of mother, foetus and newborn. This knowledge is key to enabling future health policies to optimise protection for both mother and infant, developing future vaccine scheduling recommendations, and informing professional advice. In addition, a better understanding of the benefits of influenza vaccination during pregnancy may help increase vaccination rates among pregnant women.
AUTHOR CONTRIBUTIONS
WC, NG, JF, SB, JMcV and RM designed the study, conducted the analysis and prepared the manuscript for publication. The following co‐authors provided original data and approved the final manuscript for submission: SAM, MCN, LMC, SYL, CNL, KY, HB, BC, ASC, GBR, ES and BF.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank Dr Juan‐Pablo Villanueva‐Cabezas and Dr Patricia Campbell for their assistance in revising the manuscript.
Cuningham W, Geard N, Fielding JE, et al. Optimal timing of influenza vaccine during pregnancy: A systematic review and meta‐analysis. Influenza Other Respi Viruses. 2019;13:438–452. 10.1111/irv.12649
Funding information
No funding was received to conduct this systematic review and meta‐analysis.
REFERENCES
- 1. Shahab S, Glezen W. Viral diseases in pregnancy: influenza virus. New York, NY: Springer‐Verlag; 1994:215‐223. [Google Scholar]
- 2. Kachikis A, Englund JA. Maternal immunization: Optimizing protection for the mother and infant. J Infect. 2016;72:S83‐S90. [DOI] [PubMed] [Google Scholar]
- 3. Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207(3 Suppl.):S3‐S8. [DOI] [PubMed] [Google Scholar]
- 4. Roberts S, Hollier LM, Sheffield J, Laibl V, Wendel GD. Cost‐effectiveness of universal influenza vaccination in a pregnant population. Obstet Gynecol. 2006;107(6):1323‐1329. [DOI] [PubMed] [Google Scholar]
- 5. Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;11:1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodds L, McNeil SA, Fell DB, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ: Can Med Assoc J = journal de l'Association medicale canadienne. 2007;176(4):463‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094‐1102. [DOI] [PubMed] [Google Scholar]
- 8. Yuen CY, Tarrant M. A comprehensive review of influenza and influenza vaccination during pregnancy. J Perinatal Neonatal Nurs. 2014;28(4):261‐270. [DOI] [PubMed] [Google Scholar]
- 9. Fell DB, Savitz DA, Kramer MS, et al. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG: Int J Obstetrics Gynaecol. 2017;124(1):48‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luteijn JM, Brown MJ, Dolk H. Influenza and congenital anomalies: a systematic review and meta‐analysis. Hum Reprod. 2014;29(4):809‐823. [DOI] [PubMed] [Google Scholar]
- 11. Xu J, Zhou F, Reed C, Chaves SS, Messonnier M, Kim IK. Cost‐effectiveness of seasonal inactivated influenza vaccination among pregnant women. Vaccine. 2016;34(27):3149‐3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hulka JF. Effectiveness of polyvalent influenza vaccine in pregnancy: report of a controlled study during an outbreak of Asian influenza. Obstet Gynecol. 1964;23(6):830‐837. [PubMed] [Google Scholar]
- 13. Sheffield JS, Greer LG, Rogers VL, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012;120(3):532‐537. [DOI] [PubMed] [Google Scholar]
- 14. Irving WL, James DK, Stephenson T, et al. Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG: Int J Obstetrics Gynaecol. 2000;107(10):1282‐1289. [DOI] [PubMed] [Google Scholar]
- 15. Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008;8:44‐52. [DOI] [PubMed] [Google Scholar]
- 16. Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol. 2004;21(6):333‐339. [DOI] [PubMed] [Google Scholar]
- 17. CDC . Prevention and control of influenza: recommendations of the MMWR. Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices (ACIP). Morbidity, Mortality Weekly Rep. 2004;53:1–40. [PubMed] [Google Scholar]
- 18. Committee opinion no. 305 . Influenza vaccination and treatment during pregnancy. ACOG. 2004;104(5):1125‐1126. [PubMed] [Google Scholar]
- 19. Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51(12):1355‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poehling KA, Szilagyi PG, Staat MA, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol. 2011;204(6 Suppl. 1):S141‐S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salam RA, Das JK, Dojo Soeandy C, Lassi ZS, Bhutta ZA. Impact of Haemophilus influenzae type B (Hib) and viral influenza vaccinations in pregnancy for improving maternal, neonatal and infant health outcomes. Cochrane Database Syst Rev. 2015(6):CD009982 10.1002/14651858.CD009982.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meharry PM, Colson ER, Grizas AP, Stiller R, Vazquez M. Reasons why women accept or reject the trivalent inactivated influenza vaccine (TIV) during pregnancy. Matern Child Health J. 2013;17(1):156‐164. [DOI] [PubMed] [Google Scholar]
- 23. Taksdal SE, Mak DB, Joyce S, et al. Predictors of uptake of influenza vaccination. Aust Fam Phys. 2013;42(8):582‐586. [PubMed] [Google Scholar]
- 24. McCarthy EA, Pollock WE, Nolan T, Hay S, McDonald S. Improving influenza vaccination coverage in pregnancy in Melbourne 2010‐2011. Aust N Z J Obstet Gynaecol. 2012;52(4):334‐341. [DOI] [PubMed] [Google Scholar]
- 25. McCarthy EA, Pollock WE, Tapper L, Sommerville M, McDonald S. Increasing uptake of influenza vaccine by pregnant women post H1N1 pandemic: a longitudinal study in Melbourne, Australia, 2010 to 2014. BMC Pregnancy Childbirth. 2015;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Australian Immunisation Handbook, 10 ed.: Australian Government (Department of Health); 2014.
- 27. Takeda S, Hisano M, Komano J, Yamamoto H, Sago H, Yamaguchi K. Influenza vaccination during pregnancy and its usefulness to mothers and their young infants. J Infect Chemother. 2015;21(4):238‐246. [DOI] [PubMed] [Google Scholar]
- 28. Manske JM. Efficacy and effectiveness of maternal influenza vaccination during pregnancy: a review of the evidence. Matern Child Health J. 2014;18(7):1599‐1609. [DOI] [PubMed] [Google Scholar]
- 29. McMillan M, Kralik D, Porritt K, Marshall H. Influenza vaccination during pregnancy: a systematic review of effectiveness and safety. JBI Database Syst Rev Implement Rep. 2014;12(6):281‐381. [DOI] [PubMed] [Google Scholar]
- 30. Nunes MC, Aqil AR, Omer SB, Madhi SA. The effects of influenza vaccination during pregnancy on birth outcomes: a systematic review and meta‐analysis. Am J Perinatol. 2016;33(11):1104‐1114. [DOI] [PubMed] [Google Scholar]
- 31. McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine. 2015;33(18):2108‐2117. [DOI] [PubMed] [Google Scholar]
- 32. Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018;2:CD001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Committee for proprietary medicinal products (CPMP). Guidance on harmonisation of requirements for influenza vaccines. The European Agency for the Evaluation of Medicinal Products; 1997.
- 35. Horiya M, Hisano M, Iwasaki Y, et al. Efficacy of double vaccination with the 2009 pandemic influenza a (H1N1) vaccine during pregnancy. Obstet Gynecol. 2011;118(4):887‐894. [DOI] [PubMed] [Google Scholar]
- 36. Lin SY, Wu ET, Lin CH, Shyu MK, Lee CN. The safety and immunogenicity of trivalent inactivated influenza vaccination: a study of maternal‐cord blood pairs in Taiwan. PLoS ONE. 2013;8(6):e62983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohfuji S, Fukushima W, Deguchi M, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine among pregnant women: lowered antibody response by prior seasonal vaccination. J Infect Dis. 2011;203(9):1301‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamaguchi K, Hisano M, Isojima S, et al. Relationship of Th1/Th2 cell balance with the immune response to influenza vaccine during pregnancy. J Med Virol. 2009;81(11):1923‐1928. [DOI] [PubMed] [Google Scholar]
- 39. Sedgwick P. Meta‐analyses: how to read a funnel plot. BMJ: Br Med J. 2013;346:f1342‐f1342. [Google Scholar]
- 40. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 41. Jackson LA, Patel SM, Swamy GK, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis. 2011;204(6):854‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fisher BM, van Bockern J, Hart J, et al. Pandemic influenza a H1N1 2009 infection versus vaccination: a cohort study comparing immune responses in pregnancy. PLoS ONE. 2012;7(3):e33048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chao A, Huang Y‐C, Chang Y‐L, et al. Seroprevalence of influenza A H1N1 and seroconversion of mothers and infants induced by a single dose of monovalent vaccine. Taiwanese J Obstet Gynecol. 2013;52(3):356‐359. [DOI] [PubMed] [Google Scholar]
- 44. Madhi SA, Cutland CL, Kuwanda L, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918‐931. [DOI] [PubMed] [Google Scholar]
- 45. StataCorp.. Stata statistical software: release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 46. Garcia‐Putnam R, Philips Heine R, Walter E, Rock M, Edwards K, Swamy G. Effect of timing of influenza vaccination in pregnancy on maternal and cord blood antibody titers. Am J Obstet. 2013;208(1):S43. [Google Scholar]
- 47. Kostinov M, Cherdantsev A, Smitko A, Pakhomov D, Demina E. Duration of preservation of antibodies to the flu virus in the mother‐child pairs during the vaccination of women depending on the trimester of pregnancy. Int J Biomed. 2015;5(4):179‐183. [Google Scholar]
- 48. Christian LM, Porter K, Karlsson E, Schultz‐Cherry S, Iams JD. Serum proinflammatory cytokine responses to influenza virus vaccine among women during pregnancy versus non‐pregnancy. Am J Reprod Immunol. 2013;70(1):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schlaudecker EP, McNeal MM, Dodd CN, Ranz JB, Steinhoff MC. Pregnancy modifies the antibody response to trivalent influenza immunization. J Infect Dis. 2012;206(11):1670‐1673. [DOI] [PubMed] [Google Scholar]
- 50. Bischoff AL, Følsgaard NV, Carson CG, et al. Altered response to A(H1N1)pnd09 vaccination in pregnant women: a single blinded randomized controlled trial. PLoS ONE. 2013;8(4):e56700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsatsaris V, Capitant C, Schmitz T, et al. Maternal immune response and neonatal seroprotection from a single dose of a monovalent nonadjuvanted 2009 influenza A(H1N1) vaccine a single‐group trial. Ann Intern Med. 2011;155(11):733‐741. [DOI] [PubMed] [Google Scholar]
- 52. Blanchard‐Rohner G, Meier S, Bel M, et al. Influenza vaccination given at least 2 weeks before delivery to pregnant women facilitates transmission of seroprotective influenza‐specific antibodies to the newborn. Pediatr Infect Dis J. 2013;32(12):1374‐1380. [DOI] [PubMed] [Google Scholar]
- 53. Eick AA, Uyeki TM, Klimov A, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med. 2011;165(2):104‐111. [DOI] [PubMed] [Google Scholar]
- 54. Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis. 1993;168(3):647‐656. [DOI] [PubMed] [Google Scholar]
- 55. Nunes MC, Cutland CL, Dighero B, et al. Kinetics of hemagglutination‐inhibiting antibodies following maternal influenza vaccination among mothers with and those without HIV infection and their infants. J Infect Dis. 2015;212(12):1976‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Black S, Nicolay U, Vesikari T, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30(12):1081‐1085. [DOI] [PubMed] [Google Scholar]
- 57. Murray DL, Imagawa DT, Okada DM, St Geme JW Jr. Antibody response to monovalent A/New Jersey/8/76 influenza vaccine in pregnant women. J Clin Microbiol. 1979;10(2):184‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sumaya CV, Gibbs RS. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. J Infect Dis. 1979;140(2):141‐146. [DOI] [PubMed] [Google Scholar]
- 59. Sperling RS, Engel SM, Wallenstein S, et al. Immunogenicity of trivalent inactivated influenza vaccination received during pregnancy or postpartum. Obstet Gynecol. 2012;119(3):631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Beyer WE, Palache AM, Lüchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res. 2004;103(1–2):125‐132. [DOI] [PubMed] [Google Scholar]
- 61. Hobson D, Curry RL, Beare AS, Ward‐Gardner A. The role of serum haemagglutination‐inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg. 1972;70:767‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Potter CW. Determinants of immunity to influenza in man. Br Med Bull. 1979;35(1):69‐75. [DOI] [PubMed] [Google Scholar]
- 63. Memoli MJ, Shaw PA, Han A, et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. MBio. 2016;7(2):e00417‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eberhardt CS, Blanchard‐Rohner G, Lemaître B, et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62(7):829‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Almond D. Is the 1918 influenza pandemic over? Long‐term effects of in utero influenza exposure in the post‐1940 US population. J Polit Econ. 2006;114(4):672‐712. [Google Scholar]
- 66. Gunnes N, Gjessing H, Gran J, et al., editors. Seasonal and pandemic influenza infection in pregnancy and fetal death: a Norwegian registry‐based cohort study. Options IX for the control of influenza. Chicago, IL: Norwegian Institute of Public Health; 2016. [Google Scholar]
- 67. Puck JM, Glezen WP, Frank AL, Six HR. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis. 1980;142(6):844‐849. [DOI] [PubMed] [Google Scholar]
- 68. Puleston RL, Bugg G, Hoschler K, et al. Observational study to investigate vertically acquired passive immunity in babies of mothers vaccinated against H1N1v during pregnancy. Health Technol Assess. 2010;14(55):1‐82. [DOI] [PubMed] [Google Scholar]
- 69. Zuccotti G, Pogliani L, Pariani E, Amendola A, Zanetti A. Transplacental antibody transfer following maternal immunization with a pandemic 2009 influenza A(H1N1) MF59‐adjuvanted vaccine. JAMA. 2010;304(21):2360‐2361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


