Abstract
Background
The definitive treatment for knee osteoarthritis is a total knee replacement, which results in a clinically meaningful improvement in pain and physical function. However, evidence suggests that physical activity (PA) remains unchanged after total knee replacement (TKR).
Objective
The objective of this study is to investigate the efficacy, fidelity, and safety of a physical therapist–administered PA intervention for people after TKR.
Design
This study will be a randomized controlled trial.
Setting
The setting is an outpatient physical therapy clinic.
Participants
The participants are 125 individuals who are over the age of 45 and are seeking outpatient physical therapy following a unilateral TKR.
Intervention
In addition to standardized physical therapy after TKR, the intervention group will receive, during physical therapy, a weekly PA intervention that includes a wearable activity tracking device, individualized step goals, and face-to-face feedback provided by a physical therapist.
Control
The control group will receive standardized physical therapy alone after TKR.
Measurements
The efficacy of the intervention will be measured as minutes per week spent in moderate to vigorous PA at enrollment, at discharge, and at 6 months and 12 months after discharge from physical therapy. The fidelity and safety of the intervention will be assessed throughout the study.
Limitations
Participants will not be masked, PA data will be collected after randomization, and the trial will be conducted at a single site.
Conclusions
The goal of this randomized controlled trial is to increase PA after TKR. A protocol for investigating the efficacy, fidelity, and safety of a physical therapist–administered PA intervention for people after TKR is presented. The findings will be used to support a large multisite clinical trial to test the effectiveness, implementation, and cost of this intervention.
To date, there is no cure for knee osteoarthritis, and the definitive treatment for this condition is a total knee replacement (TKR).1–3 An estimated 4 million United States (US) adults have undergone a TKR; this number represents 4% of adults over the age of 50 years.4,5 By 2020, 1.3 million TKRs are projected to be performed annually in the United States.6,7
People with knee osteoarthritis participate in low levels of physical activity (PA),8,9 which can lead to negative changes in health, including weight gain, lower-body weakness, depressive symptoms, and poor functional ability.10–12 Physical inactivity can also increase the risk of preventable medical comorbidities, including diabetes, cardiovascular disease, and premature death.13–16 While TKR is successful in relieving pain and restoring function, people remain largely sedentary after TKR.17–20 Hence, there is a critical need for a targeted intervention to increase PA in this population.17–20
PA tracking devices, such as Fitbit (Fitbit Inc, San Francisco, California), provide a unique opportunity to monitor and promote real-world PA.21–24 Steps per day is the most common metric of tracking devices and these devices can accurately estimate daily PA.24,25 The primary mode of PA for older adults is walking, making activity tracking devices ideal for this population.26 In a 2007 systematic review, Bravata et al23 found pedometers significantly increase PA by roughly 2000 steps per day. These devices are effective because they incorporate multiple behavioral change techniques, such as showing progress toward step goals, self-monitoring, and feedback—thus closely aligning with the social cognitive theory to encourage an increase in PA.27
The combination of a tracking monitor with guidance to develop a PA plan are key components to promote behavioral change,28 and have been used previously in clinical trials to increase PA in people with knee osteoarthritis.28,29 Pairing of a tracking monitor, goal setting, and feedback from a physical therapist during rehabilitation after TKR could be a low-cost and effective way to increase PA after surgery. Outpatient physical therapy is an ideal, low-cost setting30,31 for a PA intervention because physical therapists are experts at prescribing and personalizing exercises to patients and see patients for multiple encounters during an episode of care.32,33 These multiple encounters increase the likelihood of changing behavior needed to increase PA, as they allow for the individualized progression of step goals and feedback from the patient.27 At present, it is unknown whether a physical therapist–administered PA intervention after TKR can increase PA more than standardized physical therapy alone.
The purpose of this article is to present a protocol for a randomized clinical trial to investigate the efficacy, fidelity, and safety of a physical therapist–administered PA intervention for people after TKR.
The primary objective for this trial is to determine the efficacy of a physical therapist–administered PA intervention for people after TKR. Our hypothesis is that people in the intervention group will spend more minutes per week in moderate to vigorous PA (MVPA) than those in the control group at discharge from physical therapy and at 6 months and 12 months after discharge from physical therapy.
Our secondary objective is to evaluate the fidelity and safety of our intervention and investigate changes in self-reported and performance-based physical function. We hypothesize that people in the intervention group will have similar baseline-to-discharge changes in self-reported physical function and objective physical function as the control group.
The exploratory objective is to evaluate the association of psychosocial factors with PA at 6 months and 12 months after discharge from physical therapy. We hypothesize that people with low levels of self-efficacy for exercise and high kinesiophobia will have lower levels of PA.
This is a single center, randomized clinical trial comparing standardized physical therapy with a physical therapist–administered PA intervention after unilateral TKR. This study is registered at ClinicalTrials.gov (trial no. NCT03228719). The trial will follow the CONSORT guidelines for participant enrollment and tracking and the TIDieR checklist to describe the intervention (Fig.).
Figure.
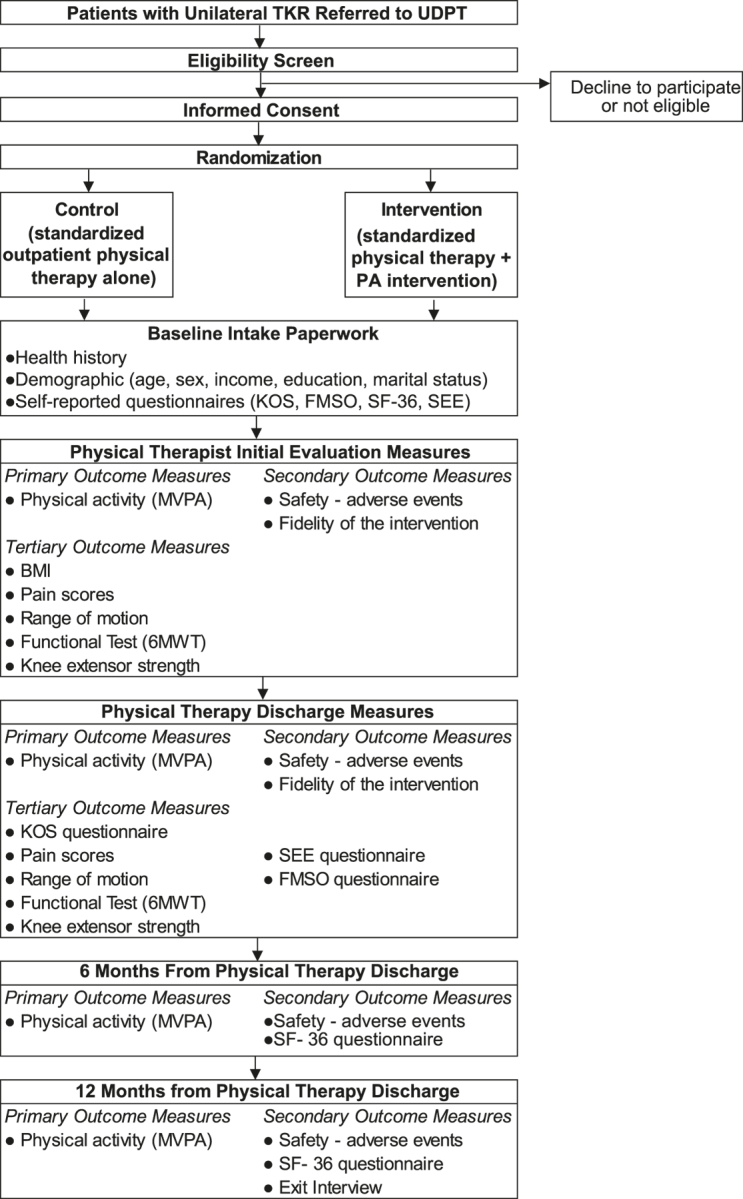
Flow chart. 6MWT = 6-Minute Walk Test, FMSO = Fear of Movement Scale for Osteoarthritis questionnaire, KOS = Knee Outcome Scale, MVPA = moderate-to-vigorous physical activity, PA = physical activity, SEE = Self-Efficacy for Exercise, SF-36 = Medical Outcomes Study (MOS) 36-Item Short Form, TKR = total knee replacement, UDPT = Delaware Physical Therapy Clinic at the University of Delaware.
Methods
Participants
Physical therapists at the Delaware Physical Therapy Clinic at the University of Delaware (UDPT) will recruit participants who are referred to physical therapist for a unilateral TKR. Physical therapists will inform their patient of potential eligibility for our study. If the patient is interested, then a research assistant will determine eligibility, provide pertinent study information, and obtain written informed consent from each participant in person.
Participants who are older than 45 years, have not had or are not planning another lower extremity surgery within 6 months of the study, are seeking outpatient physical therapist services for a unilateral TKR, are interested in increasing their PA, and have not participated in a PA intervention study at UDPT are eligible to participate in this study. Participants are excluded if they have any additional comorbidities that would not allow them to participate in a PA intervention or do not meet the inclusion criteria.
Randomization and Masking
After enrollment in the study, participants are randomized into a control or an intervention group. During the informed consent process, all participants will receive information about the control and intervention group prior to randomization. Participants will not be masked to their group assignments because of the nature of our intervention and the pragmatic design of our trial. A computer randomizer (IBM SPSS Statistics for Windows, v 23 [IBM Corp, Armonk, New York]) will be used to randomize the 125 participants. A research assistant will use the randomizer to assign the participants to a group and provide the participant with study documentation. All other members of the research team will be masked to participants’ group assignments. The treating physical therapists, however, will not be masked to the group assignments.
Data Collection and Management
PA data will be collected using an accelerometer for 1 week after enrollment, at discharge from physical therapy, and at 6 months and 12 months after discharge from physical therapy. Research assistants will gather additional demographic, medical, insurance, and clinical data for analysis through medical chart review and will enter data into an electronic database developed by Delaware Rehabilitation Institute and securely stored on a server.
Control Group
Participants in the control group will receive standardized physical therapy provided by a licensed physical therapist at UDPT using the University of Delaware's Rehabilitation Guidelines for Unilateral Total Knee Replacement (eAppendix 1, available at https://academic.oup.com/ptj). Standardized physical therapy also includes a printed home exercise program with an exercise log that is updated weekly by a physical therapist (eAppendix 2).
Intervention
The intervention group will receive a PA intervention in addition to the same standardized physical therapy as the control group. The PA intervention consists of a Fitbit Zip, individualized step goal, and face-to-face feedback provided by a physical therapist.
Fitbit Zip
A research assistant will provide participants in the intervention group with a Fitbit Zip within 1 week after enrolling in the study. They will be asked to wear their Fitbit Zip daily at their right hip during waking hours. They will be asked to remove the device during activities in which the device could get wet (eg, showering, swimming). Participants will be given written and in-person instructions on how to sync their Fitbit Zip to their personal smartphone, tablet, or home computer to track their steps per day.
Individualized step goal
Participants will self-track their steps per day using their Fitbit Zip. The step-per-day goal will be based on the data from the Fitbit Zip. They will use their personal electronic device to either show the physical therapist in-person via the Fitbit app or have a written log of their weekly steps per day. When participants are in phase 1 of the UDPT rehabilitation guidelines, 0 to 2 weeks after TKR, they are not expected to increase their PA above 10 minutes per day. Beyond 3 weeks after surgery, phase 2 of the UDPT rehabilitation guidelines, step goals will be initiated and evaluated on a weekly basis. Progression of the weekly step-per-day goal will be a joint decision between the participant and physical therapist. As a guide, the physical therapist will instruct the participant to target a 10% to 20% increase in steps per day until at least 6000 steps per day are achieved. Each week the physical therapist will review the participant's step-per-day goal, and if at least 4 of the 7 previous days per week were at or above the prior week's step-per-day goal, then next week's step-per-day goal will be incrementally increased. This regimen will continue over the course of their rehabilitation, with the end goal of at least 6000 steps per day at discharge from physical therapy. A participant who walks at least 6000 steps per day will be encouraged to continue to increase PA beyond 6000 steps per day without a ceiling threshold because the benefits of PA are not limited but continue in a dose-response fashion.
Face-to-face feedback
The physical therapist will discuss current steps per day with the participant using the Fitbit Zip and set a personalized step-per-day goal each week during their standardized outpatient physical therapy rehabilitation. Physical therapists will encourage participants to meet their step-per-day goal the majority of days during the week while discussing the number of steps per day achieved by the participant. This process will take less than 5 minutes and occur during the participant's physical therapist visit.
Outcome Measures
Primary outcome
Our primary outcome is PA, specifically, minutes per week in MVPA. Increasing MVPA by more than 30 minutes per week is associated with a reduction in risk associated with future mortality and morbidity.34 Therefore, by using an increase of 30 minutes per week in MVPA as our primary outcome measure, we can capture health gains associated with PA after TKR. We hypothesize that the intervention group will spend 30 minutes more per week in MVPA than the control group at each follow-up time point. We will measure MVPA using an accelerometer, which is a valid and reliable measure of daily PA in older adults.35,36 PA will be measured at 4 time points: baseline, discharge from physical therapy, and 6 months and 12 months after discharge from physical therapy. We will use an Actigraph GT3X (Actigraph, Pensacola, Florida), to measure PA. The participant will be instructed, in person, to wear the monitor around the waist with the accelerometer located at the right hip. A research assistant will instruct participants to put on the monitor when they get up in the morning, take off the monitor when they go to sleep in the evening, and remove the monitor during situations in which the device could get wet (eg, showering, swimming). The Actigraph GT3X will be worn for 1-week bouts at each time point. A masked research assistant will download, screen, and process data from the Actigraph GT3X using a standardized protocol.36 Specifically, we will include data collected from the monitor when it was worn for more than 4 valid days. A valid day is defined as having more than 10 hours of wear time.36
Secondary outcome measures
Our secondary outcome measures include intervention fidelity, that is, measuring the adherence of implementing the PA intervention in physical therapist practice. We will check whether physical therapists documented that they reviewed a participant's PA using the Fitbit and whether they reviewed with the participant the individualized step goal in the home exercise program log. Intervention fidelity will be tracked from enrollment to discharge from physical therapy.
The safety of the intervention will be evaluated by measuring adverse events that occur from enrollment to 12 months. An adverse event is defined as any unfavorable or unintended diagnosis, sign, symptom, or disease that is associated with the study and that may or may not be related to the intervention. Preenrollment adverse events will not be included in the safety assessment.
Self-reported physical function will be measured using the Knee Outcome Survey and the Physical Component Summary of the 36-Item Short-Form Survey. The Knee Outcome Survey is a valid and reliable measurement of patient-reported function of the knee, and participants will fill this form out to measure their function after TKR.37 The Physical Component Summary of the 36-Item Short-Form Survey is routinely used in practice and is a valid and reliable measure of the quality of life after TKR.38
The objective measure of physical function will be walking endurance on the 6-minute walk test, which is a reliable and valid measure of community ambulation in people with TKR.39
These measures will be collected via medical chart review by a research assistant. Data will be gathered at the initial physical therapist evaluation and at discharge from physical therapy.
Exploratory outcome measures
The Self-Efficacy for Exercise (SEE) survey and the Fear of Movement Scale for Osteoarthritis (FMSO) questionnaire. The SEE survey is a valid and reliable measure of self-efficacy for exercise in older adults,40 and the FMSO questionnaire is a valid and reliable measure of fear related to movement in people with osteoarthritis.41 Both the SEE survey and the FMSO questionnaire will be used at enrollment and at discharge from physical therapy.
Additional data will be collected through the participant's health history: age, sex, body mass index, level of education, household income, marital status, range of motion, knee extension strength, and knee pain on a numerical pain rating scale at best, at worst, and with movement. These data will be used to evaluate for differences between groups at baseline and to adjust the models as needed.
Exit interview
At the 12-month time point, a research assistant will read several scripted exit interview questions to participants on the basis of their group assignments (eAppendix 3, available at https://academic.oup.com/ptj).
Data Analysis, Monitoring, and Auditing
Sample size calculation
Our primary outcome measure is MVPA. We hypothesize that the intervention group will spend 30 minutes per week more in MVPA than the control group, with a standard deviation of 60 minutes per week. In a randomized controlled trial, Bossen et al42 reported a 24-minute difference in MVPA at 12 months between the groups. We used this change in MVPA as a basis for our hypothesis. We will need a sample size of 125 to achieve 5% of a type I error and 80% power to reach statistical significance, assuming a 20% dropout rate. In addition, we will use an alpha level of .05 and a 2-tailed test in order to determine a difference between groups for MVPA.
Statistical analysis
We will describe primary, secondary, and outcome measures for both the intervention and control groups using means and standard deviations for continuous measures and frequencies for categorical measures. To ensure groups are equal at baseline, we will compare demographics and participant characteristics at enrollment using an analysis of variance or chi-squared test. If the groups are different at baseline, then we will adjust for the variables in a multivariate model. For missing baseline data, we will conduct a complete analysis with an alpha level set at .05.
To determine the efficacy of the intervention and which group spent more minutes per week in MVPA, we will use a 2-way mixed-design analysis of variance and compare PA at discharge from physical therapy and PA at 6 months and 12 months after discharge from physical therapy. We will analyze the data using an unadjusted and adjusted multiple regression model, adjusting for potential confounders including age, sex, body mass index, education, comorbidity, knee pain intensity, and depressive symptoms. Analyses will be run twice, first using a per-protocol analysis that tests the data for all those who completed all sessions. Second, we will evaluate whether the added component (addition of the intervention) affected results by running the analysis again using an intention-to-treat approach with a last-value-carried-forward method. If dropouts exceed the expected 20%, then analysis will be done using a general linear mixed-methods model.
To analyze the secondary outcomes, fidelity, and safety of the intervention, we will describe in terms of frequencies (ie, the percentage of physical therapist appointments that included both review of the participant's PA and review of the individualized step goal from the electronic medical record). We will report the mean and standard deviation of the occurrences. Adverse events will also be reported in a similar manner. Finally, to explore the association of self-efficacy for exercise and kinesiophobia with the intervention, we will calculate the effect estimates from a linear model with interaction by group assignment and adjust for the same potential confounders.
Ethics
Ethics approval was obtained from the Internal Review Board at the University of Delaware, Human Subjects Research Committee (946,165-5).
Role of the Funding Source
This study was partially funded by the National Institutes of Health (NIH ref. nos. 1R21AR071079-01A1, NIH 5 U54 GM104941-3, and NIH 5 K12 HD055931-09), which had no involvement in the drafting or approval of this article.
Discussion
Our project is important because we are investigating a physical therapist–administered PA intervention in a population prone to persistent inactivity. If the results from this study demonstrate efficacy, as defined by the intervention group spending more time in MVPA, as well as fidelity and safety of the intervention, then we will proceed to a large multisite trial. This intervention is important because it has the potential to increase PA and mitigate future disability in people after TKR.
Strengths and Weaknesses
The strength of our study is that it is a randomized controlled trial, powered to determine the efficacy of a physical therapist–administered PA intervention. In addition, the intervention is pragmatic and physical therapists have the ability to make a patient-centered clinical decision to develop and progress the activity intervention.
A weakness of our study is that participants will not be masked to their group assignments because of our pragmatic trial design and the type of PA intervention that we used. Another limitation of our study is that PA data collection happens after randomization. The study is taking place in a clinical setting, and, for logistical purposes, we are unable to collect PA data before randomization. We did not exclude participants who currently use or plan on using a PA monitor during their outpatient physical therapy after TKR. This could result in contamination of the control—for example, participants in the control group using an activity monitor. Since the control group will not receive the step goals, we believe there will be minimal to no effect of contamination on our study outcome. Single-site for our trial is also a weakness, since it may limit generalizability to other clinics.
Contribution to the Physical Therapist Profession
Pending the results of our study, we aim to change physical therapist clinical practice by targeting PA as a part of routine treatment after TKR. Traditionally, physical therapy after TKR addresses impairments and functional limitations—such as pain or the ability to rise from a chair—with little attention related to PA. During outpatient physical therapy, there is a teachable moment, and our intervention intends to use this moment to address physical inactivity and improve health in this patient population.
Supplementary Material
Author Contributions and Acknowledgments
Concept/idea/research design: L. Thoma, H. Master, L. Schmitt, R. Pohlig, D.K. White
Writing: M.B. Christiansen, L. Thoma, H. Master, L. Schmitt, R. Pohlig, D.K. White
Data collection: M.B. Christiansen, L. Schmitt
Data analysis: H. Master, R. Pohlig, D.K. White
Project management: M.B. Christiansen, L. Thoma, H. Master, L. Schmitt
Fund procurement: D.K. White
Providing participants: L. Schmitt
Providing facilities/equipment: L. Schmitt, D.K. White
Providing institutional liaisons: L. Schmitt
Clerical/secretarial support: L. Schmitt
Consultation (including review of manuscript before submitting): L. Thoma, L. Schmitt, R. Pohlig
M.B.C. drafted the study protocol as first author and assisted in the development of the trial design. D.K.W developed the study design, critically revised the manuscript, and is principal investigator of this study. L.M.T. critically revised the manuscript and contributed to the trial design. H.M. and R.P. contributed to the statistical analysis section and with the development of the statistical plan. L.A.S. assisted with participant enrollment, consenting, and data acquisition plan.
The authors thank their colleagues at the Delaware Physical Therapy Clinic, University of Delaware, for helping them set up this project. In addition, they thank patients for contributing to the development of this trial by enrolling and participating in the pilot study.
Ethics Approval
Ethics approval was obtained from the Internal Review Board at the University of Delaware, Human Subjects Research Committee (ref. no. 946,165-5).
Funding
This study was partially funded by the National Institutes of Health (NIH ref nos. 1R21AR071079- 01A1, NIH 5 U54 GM104941-3, and NIH 5 K12 HD055931-09).
Clinical Trial Registration
This study is registered at ClinicalTrials.gov (trial no. NCT03228719).
Disclosure
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Hunter DJ, Felson DT. Osteoarthritis. J Med Internet Res. 2006;332:862–871. [Google Scholar]
- 2. Fibel KH, Hillstrom HJ, Halpern BC. State-of-the-art management of knee osteoarthritis. World J Clin Cases. 2015;3:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dowsey MM, Gunn J, Choong PFM. Selecting those to refer for joint replacement: who will likely benefit and who will not? Best Pract Res Clin Rheumatol. 2014;28:157–171. [DOI] [PubMed] [Google Scholar]
- 4. Weinstein AM, Rome BN, Reichmann WM et al. . Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. Bone Joint J. 2005;87:1487–1497. [DOI] [PubMed] [Google Scholar]
- 6. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Bone Joint J. 2007;89:780–785. [DOI] [PubMed] [Google Scholar]
- 7. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. Bone Joint J. 2014;96:624–630. [DOI] [PubMed] [Google Scholar]
- 8. Wallis JA, Webster KE, Levinger P, Taylor NF. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthritis Cartilage. 2013;21:1648–1659. [DOI] [PubMed] [Google Scholar]
- 9. Herbolsheimer F, Schaap LA, Edwards MH et al. . Physical activity patterns among older adults with and without knee osteoarthritis in six European countries. Arthritis Care Res. 2016;68:228–236. [DOI] [PubMed] [Google Scholar]
- 10. Lee J, Song J, Hootman JM et al. . Obesity and other modifiable factors for physical inactivity measured by accelerometer in adults with knee osteoarthritis: data from the Osteoarthritis Initiative (OAI). Arthritis Care Res. 2013;65:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ettinger WH, Davis MA, Neuhaus JM, Mallon KP. Long-term physical functioning in persons with knee osteoarthritis from NHANES I: effects of comorbid medical conditions. J Clin Epidemiol. 1994;47:809–815. [DOI] [PubMed] [Google Scholar]
- 12. Bindawas SM, Vennu V. Longitudinal effects of physical inactivity and obesity on gait speed in older adults with frequent knee pain: data from the Osteoarthritis Initiative. Int J Environ Res Public Health. 2015;12:1849–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hawker GA, Croxford R, Bierman AS et al. . All-Cause mortality and serious cardiovascular events in people with hip and knee Osteoarthritis: A population based cohort study. PLoS One. 2014;9:e91286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piva SR, Susko AM, Khoja SS, Josbeno DA, Fitzgerald GK, Toledo FG. Links between osteoarthritis and diabetes: implications for management from a physical activity perspective. Clin Geriatr Med. 2015;31:67–87, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shortreed SM, Peeters A, Forbes AB. Estimating the effect of long-term physical activity on cardiovascular disease and mortality: evidence from the Framingham Heart Study. Heart. 2013;99:649–654. [DOI] [PubMed] [Google Scholar]
- 17. Paxton EW, Torres A, Love RM, Barber TC, Sheth DS, Inacio MCS. Total joint replacement: a multiple risk factor analysis of physical activity level 1–2 years postoperatively. Acta Orthop. 2016;87:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammett T, Simonian A, Austin M et al. . Changes in physical activity after total hip or knee arthroplasty: A systematic review and meta-analysis of 6 and 12 month outcomes. Arthritis Care Res (Hoboken). 2017. [DOI] [PubMed] [Google Scholar]
- 19. Vogel LA, Carotenuto G, Basti JJ, Levine WN. Physical activity after total joint arthroplasty. Sports Health. 2011;3:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paxton RJ, Melanson EL, Stevens-Lapsley JE, Christiansen CL. Physical activity after total knee arthroplasty: a critical review. World J Orthop. 2015;6:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coughlin SS, Stewart J. Use of consumer wearable devices to promote physical activity: a review of health intervention studies. J Environ Health Sci. 2016;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talbot LA, Gaines JM, Huynh TN, Metter EJ. A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: a preliminary study. J Am Geriatr Soc. 2003;51:387–392. [DOI] [PubMed] [Google Scholar]
- 23. Bravata DM, Smith-Spangler C, Sundaram V et al. . Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. [DOI] [PubMed] [Google Scholar]
- 24. Berlin JE, Storti KL, Brach JS. Using activity monitors to measure physical activity in Free-Living conditions. Phys Ther. 2006;86:1137–1145. [PubMed] [Google Scholar]
- 25. Almeida GJ, Irrgang JJ, Fitzgerald GK, Jakicic JM, Piva SR. Reliability of physical activity measures during free-living activities in people after total knee arthroplasty. Phys Ther. 2016;96:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yusuf HR, Croft JB, Giles WH et al. . Leisure-time physical activity among older adults: United States, 1990. Arch Intern Med. 1996;156:1321–1326. [PubMed] [Google Scholar]
- 27. Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Medical Internet Res. 2014;16:e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li LC, Sayre EC, Xie H, Clayton C, Feehan LM. A community-based physical activity counselling program for people with knee osteoarthritis: feasibility and preliminary efficacy of the Track-OA Study. JMIR Mhealth Uhealth. 2017;5:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones D, Skrepnik N, Toselli RM, Leroy B. Incorporating novel mobile health technologies into management of knee osteoarthritis in patients treated with intra-articular hyaluronic acid: rationale and protocol of a randomized controlled trial. JMIR Res Protoc. 2016;5:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterson LE, Goodman C, Karnes EK, Chen CJ, Schwartz JA. Assessment of the quality of cost analysis literature in physical therapy. Phys Ther. 2009;89:733–755. [DOI] [PubMed] [Google Scholar]
- 31. Bürge E, Monnin D, Berchtold A, Allet L. Cost-effectiveness of physical therapy only and of usual care for various health conditions: systematic review. Phys Ther. 2016;96:774–786. [DOI] [PubMed] [Google Scholar]
- 32. Dean E. Physical therapy in the 21st century (part II): evidence-based practice within the context of evidence-informed practice. Physiother Theory Pract. 2009;25:354–368. [DOI] [PubMed] [Google Scholar]
- 33. Dean E. Physical therapy in the 21st century (part I): toward practice informed by epidemiology and the crisis of lifestyle conditions. Physiother Theory Pract. 2009;25:330–353. [DOI] [PubMed] [Google Scholar]
- 34. Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol. 2012;2012:718789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song J, Semanik P, Sharma L et al. . Assessing physical activity in persons with knee osteoarthritis using accelerometers: data in the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2010;62:1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. [DOI] [PubMed] [Google Scholar]
- 37. Impellizzeri FM, Mannion AF, Leunig M, Bizzini M, Naal FD. Comparison of the reliability, responsiveness, and construct validity of 4 different questionnaires for evaluating outcomes after total knee arthroplasty. J Arthroplasty. 2011;26:861–869. [DOI] [PubMed] [Google Scholar]
- 38. Escobar A, Quintana JM, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage. 2007;15:273–280. [DOI] [PubMed] [Google Scholar]
- 39. Jakobsen TL, Kehlet H, Bandholm T. Reliability of the 6-min walk test after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:2625–2628. [DOI] [PubMed] [Google Scholar]
- 40. Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise scale. Nurs Res. 2000;49:154–159. [DOI] [PubMed] [Google Scholar]
- 41. Shelby RA, Somers TJ, Keefe FJ et al. . Brief Fear of Movement Scale for osteoarthritis. Arthritis Care Res (Hoboken). 2012;64:862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bossen D, Veenhof C, Van Beek KE Spreeuwenberg PM, Dekker J, De Bakker DH. Effectiveness of a web-based physical activity intervention in patients with knee and/or hip osteoarthritis: randomized controlled trial. J Med Internet Res. 2013;15:e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


