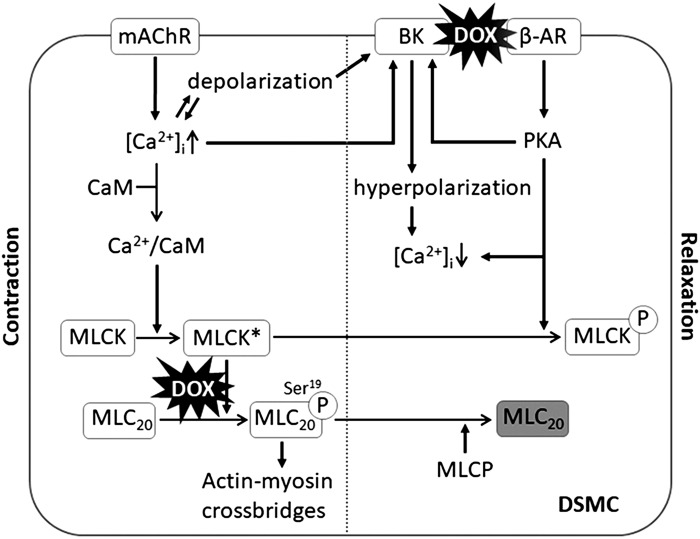
Fig. 7.
Schematic diagram of the proposed mechanisms underlying doxorubicin (DOX)-induced detrusor smooth muscle (DSM) dysfunction. An activation of the muscarinic acetylcholine receptor (mAChR) triggers an increase in intracellular Ca2+ concentration ([Ca2+]i), leading to Ca2+/calmodulin (CaM)-dependent activation of myosin light chain kinase (MLCK*). MLCK* phosphorylates myosin light chain 20 (MLC20), which enables the forming of actin-myosin cross-bridges, leading to muscle contraction. DOX causes a decrease in MLC20 phosphorylation, resulting in a reduction of muscle contractility (left). Increased Ca2+, cell depolarization, and β-adrenoceptor (β-AR) pathway activate the large-conductance Ca2+-activated K+ (BK) channel, which causes K+ efflux and hyperpolarizes membrane potential, blocking voltage-gated Ca2+ channels, leading to a decrease in [Ca2+]i. A decreased level of phosphorylated MLC20 by myosin light-chain phosphatase (MLCP) and deactivation of myosin light-chain kinase (MLCK) via the β-AR/PKA pathway and a low level of intracellular Ca2+ lead to muscle relaxation (right). DOX causes a downregulation of BK channel activity, leading to an impairment of muscle relaxation responses. DSMC, DSM cell; encircled P, phosphorylation of the proteins MLC20 and MLCK.