Abstract
Calcific aortic valve disease (CAVD) is highly prevalent and has no pharmaceutical treatment. Surgical replacement of the aortic valve has proved effective in advanced disease but is costly, time limited, and in many cases not optimal for elderly patients. This has driven an increasing interest in noninvasive therapies for patients with CAVD. Adaptive immune cell signaling in the aortic valve has shown potential as a target for such a therapy. Up to 15% of cells in the healthy aortic valve are hematopoietic in origin, and these cells, which include macrophages, T lymphocytes, and B lymphocytes, are increased further in calcified specimens. Additionally, cytokine signaling has been shown to play a causative role in aortic valve calcification both in vitro and in vivo. This review summarizes the physiological presence of hematopoietic cells in the valve, innate and adaptive immune cell infiltration in disease states, and the cytokine signaling pathways that play a significant role in CAVD pathophysiology and may prove to be pharmaceutical targets for this disease in the near future.
Keywords: adaptive immunity, aortic valve, calcific aortic valve disease
INTRODUCTION
Calcific aortic valve disease (CAVD) affects one of four people over 65 yr of age and is the primary cause of aortic stenosis (96, 164). This prevalent and insidious disease inevitably leads to surgical and transcatheter replacement of the valve, as it has no pharmaceutical treatment. However, because the incidence of clinical aortic stenosis begins to grow exponentially after 55 yr of age, many of those affected are not optimal surgical candidates (35). This has led to great interest in discovery of new drug targets or treatment strategies earlier in the disease course.
Drug development demands an understanding of the basic science and pathophysiology of disease. In the case of CAVD, this pathophysiology is a fibrocalcific process involving myofibroblast activation, osteoblastic transition, lipoprotein deposition, and inflammation (96, 140, 141). Considering these characteristics, it is not surprising that lymphocytic infiltration defines CAVD; however, most pharmaceutical strategies have focused on general cardiovascular health with treatment for hypertension, diabetes, and dyslipidemia (96). As our general understanding of cardiovascular disease changes, and trials like the Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) attempt to utilize immune modulation in treatment of other cardiovascular diseases (144, 145), the role of immune cells in the development of CAVD is just emerging. Over the past 10 years, the literature has rapidly expanded concerning the immune signaling and cellular changes in CAVD. Here, we summarize the innate and adaptive immune cell infiltrate characteristic of CAVD, the role of cytokines in cellular calcification, and the potential role of these known signaling pathways in linking the hematopoietic cell infiltration and resident cell calcification that are concurrent in CAVD.
CELLULAR COMPOSITION OF THE AORTIC VALVE
Aortic Valve Resident Cells
To understand the impact of immune cell signaling in the the aortic valve (AV), it is necessary to understand the cellular composition of the healthy valve. The AV classically consists of two resident cell populations: aortic valve interstitial cells (AVICs) and aortic valve endothelial cells (AVECs). AVECs line the interface of the valve with the circulating blood and are embryonically derived from the secondary heart field (172, 179). AVICs are fibroblast-like cells derived from AVECs and the cardiac neural crest that make up the bulk of the valve and serve as the primary source of cellular calcification (95, 179).
Hematopoietic Cells
In the past decade, the presence of leukocytes in the healthy AV has also been described and is being slowly incorporated into calcification models. Surprisingly, up to 10–15% of valve cells are CD45+, a marker of the hematopoietic lineage (68). This fraction grows throughout maturation and is split primarily between CD133+ cells (bone marrow-derived progenitor cells) and CD11c+/molecular histocompatibility complex II+ (MHC II+) dendritic-like cells (61). Importantly, MHC II is the primary vehicle of antigen presentation for external antigens. Antigen presentation leads to T cell recognition of the antigen and is a primary step in the adaptive immune response. Choi et al. (32) first identified CD11c+ cells with dendritic processes in the AV and further showed that their aortic wall counterparts 1) highly express MHC II and moderately express CD11c and CD86 (a costimulatory molecule that, in conjunction with antigen presentation, promotes T cell activation) at a population level and 2) could proficiently present ovalbumin to T cells. These characteristics explicitly confirm the presence of functional antigen-presenting cells (APCs) in the AV. The most common APCs are dendritic cells, macrophages, and B cells. It has been shown that the APCs in the valve variably express the macrophage markers CD206 and F4/80 (68), suggesting, in concert with the above data, that they may be primarily macrophages.
In physiological states, these cells serve as immune surveillance cells. Namely, they phagocytose pathogens and traffic to the lymphatic system, in which they present antigens and initiate immune responses. To that end, Hajdu et al. (61) have shown that the hematopoietic cells in the healthy valve are constantly being replaced, as is common of immune surveillance cells in many tissues. In the healthy valve, these cells would serve to initiate immune responses to valvular endocarditis but otherwise play a more understated role through local juxtacrine or paracrine signaling in the absence of offending pathogens.
MYELOID CELL INFILTRATE DEFINES CAVD
Antigen-Presenting Cells in CAVD
Although the presence of immune cells in the healthy valve is a new finding, leukocytic infiltrates have been observed in CAVD for at least 25 years (Table 1), first noted in the calcification of porcine bioprostheses after AV replacement (132). Nonetheless, any roles of these cells in CAVD pathophysiology are largely unknown (90). As the fields of cardiology and immunology have increasingly overlapped, initially in the understanding of atherosclerosis (54), followed by hypertension (129), a renewed focus has emerged on the role of infiltrating immune cells in CAVD.
Table 1.
Hematopoietic cells identified in the valve
| Cell Type | Immunological Role | Identifiers | Notes | Referencess |
|---|---|---|---|---|
| In the healthy valve (murine unless otherwise noted) | ||||
| Dendritic cells | Initiate innate immune response, antigen-presentation | CD45+, CD11c+, F4/80− | ||
| CD11b+ dendritic cells | CD11b+ | (32) | ||
| CD11b− dendritic cells | CD11b− | (68) | ||
| Macrophages | Phagocytose pathogens, initiate innate immune response, antigen-presentation | CD11c+, F4/80+ | (9, 68) | |
| MHC II+ macrophages | MHC II+ | (68) | ||
| CD206+ macrophages | CD206+ | Murine | (68) | |
| CD68+, CD206+ | Human | (92) | ||
| Enriched in CAVD (human unless otherwise noted) | ||||
| Myeloid cells | ||||
| Dendritic cells | Initiate innate immune response, antigen-presentation | S100A9+ | (121, 157) | |
| Macrophages | Phagocytose pathogens, initiate innate immune response, antigen-presentation | CD68+ | (36, 93, 112, 157, 162) | |
| M1-like macrophages | Initiate inflammatory and cytotoxic immune responses | CD11c+ | ↑ in CAVD | (92) |
| M2-like macrophages | Initiate tolerogenic and profibrotic immune responses | CD206+ | ↓ in CAVD | (92) |
| Lymphocytes | ||||
| T cells | CD3+, IL2R+, CD6+ | (36, 112, 132, 157) | ||
| T helper cells | Coordinate the immune response by providing “help” to other cells | CD4+ | (121, 162) | |
| CTLs | Kill infected cells or tumor cells | CD8+, IFNγ+ | (123, 162) | |
| Regulatory T cells | Resolve immune response, promote tolerogenic environment | CD25+, Foxp3+ | In circulation; ↓ after surgical intervention | (155) |
| Memory-effector T cells | Initiate immune responses to prior pathogens | CD8+, CD28null, CD57+ | In both valve and circulation | (178) |
| Natural killer T cells | Release cytokines in response to various glycolipid antigens | ↑ with worsening echo metrics | (111) | |
| B cells | CD20+, BAFF-R+ | (126, 162) | ||
| Plasma cells | Produce antibody | CD138+ | (162) | |
| Other | ||||
| Mast cells | Rapidly release histamines and inflammatory substances during allergic reactions | CD117+, tryptase+, chymase+ | ↑ in bicuspid aortic valves; increased with worsening echo | (119, 163, 183) |
| Osteogenic progenitor cells | Bone formation | OCN+, CD34+, KDR+ | ↑ with worsening echo; in both valve and circulation | (44, 55, 167) |
CAVD, calcific aortic valve disease; CTLs, cytotoxic T lymphocytes; MHC II, molecular histocompatibility complex II; OCN, osteocalcin; IFNγ, interferon-γ; Foxp3, forkhead box P3; BAFF-R, B cell-activating factor receptor; KDR, kinase insert domain receptor.
APCs are at the crux of this new focus. APCs play a major role in defining the innate immune response to an insult and present antigen to T cells, initiating the adaptive immune cell response. As outlined above, APCs such as macrophages and dendritic cells are present in the healthy valve. This has called attention to their potential role in the pathogenesis of CAVD. Notably, in the calcified valve there is an enrichment of the macrophage population (36, 112, 157) and an increase of CD11c+ as opposed to CD206+ macrophages (92). CD11c positivity is a marker of inflammatory M1-like macrophages, which are generally responsive to interferon-γ (IFNγ) and lipopolysaccharide (LPS) activation, efficiently secrete IL-12, tumor necrosis factor-α (TNFα), and other acute phase reactants, and direct a proinflammatory immune response (104, 105). In contrast, CD206 is a marker of M2 macrophages, which generally promote immunoregulation and long-term antibody production (108). Although the M1/M2 macrophage model is a simplified dichotomization, the general concept of inflammatory and tolerogenic or immunosuppressive macrophages is useful here: an increase in M1 polarization like that found in CAVD by Li et al. (92) represents a heightened inflammatory state. Similarly, an increase in transcripts of human leukocyte antigen (HLA) subtypes and other proteins involved in antigen presentation in calcified valves reinforces the concept of increased inflammatory activity of APCs in the pathophysiology of CAVD (152).
Mechanistic investigations have provided mixed results on the role of APCs in the calcification process. AVICs treated with conditioned media from M1 macrophages increase expression of osteogenic calcification markers (bone morphogenetic protein-2, osteopontin, and alkaline phosphatase) at the mRNA and protein levels (92), and antibody blockade of inflammatory cytokines TNFα and/or IL-6 decreases this calcification effect in vitro. This would suggest a procalcific role for macrophages. However, nonspecific depletion of macrophages in hyperlipidemic mice with liposomal clodronate leads to increased valvular thickness due to expanding lipid and collagen deposits (27). This stresses the importance of macrophage polarization and selective inhibition, as clodronate treatment depletes both M1 and M2 macrophages, which may not affect the balance between tolerogenic and inflammatory responses.
There is also a potential role for macrophages in interaction with the unique mechanical environment of the AV. Data on CAVD in the bicuspid AV (BAV) and in those with hypertension suggest a role for mechanical stimulation of APCs. Increased mechanical strain has been implicated in CAVD and is characteristic of both BAV and hypertension (51, 79, 166, 186). Cyclic mechanical strain similar to that experienced in the valve has been shown to increase macrophage activation and inflammation in multiple models (13, 138, 176, 185). Calcified BAVs, which exhibit greater mechanical strain, have significantly increased expression of antigen presentation proteins versus calcified tricuspid valves (133) and are more likely than calcified tricuspid valves to have increased macrophage infiltrate (3). Additionally, BAVs have a predominance of M1-like macrophages (175). Together, these data suggest that mechanical strain may play a role in macrophage activation and resultant inflammation in CAVD.
Other Myeloid Cells in CAVD
Two cell types that each make up a small fraction of immune cells have also been investigated in CAVD. First, mast cells, basophilic leukocytes involved in allergic responses, are present in the calcified valve and are associated with BAV (163), increased infiltration of macrophages and lymphocytes (119), and increased AV peak jet velocity (183), a common clinical marker of aortic stenosis severity. Mast cells are capable of releasing large amounts of cytokines in response to stimulus, and are responsible for systemic allergic reactions. The clinical picture of CAVD does not fit this description, but perhaps the accumulation of mast cells and the calcification of the AV are disparate but parallel processes occurring in response to a systemic stimulus. Additionally, mast cells characteristically secrete chymase, which converts angiotensin I to angiotensin II (65). Angiotensin II has been found in stenotic valves and is capable of promoting valvular thickening in mice (52, 65). Thus, it is possible that mast cells play a crucial role in the inflammation of CAVD.
Second, osteogenic progenitor cells (CD34+/osteocalcin+) have been identified in and associated with CAVD (44, 55, 167). These cells are bone marrow derived and, like osteoblasts, have bone-forming capacity (137). It is known that macrophages and osteoblasts play a synergistic role in physiological skeletal bone formation and diseases of heterotopic ossification (7, 78), and it is possible that CAVD exhibits a similar phenomenon, although it is yet to be described. Curiously, these osteogenic progenitor cells decrease with age in the general population (58), opposing the age-dependent nature of CAVD and risk factors like hypertension and atherosclerosis. Nonetheless, the remaining cells in circulation could be entering the valve during the calcification process.
LYMPHOCYTIC INFILTRATE SUGGESTS A ROLE FOR THE ADAPTIVE IMMUNE SYSTEM IN CAVD
T Lymphocytes in CAVD
Although APCs and specifically macrophages are present in both the healthy and diseased valve, T lymphocytes are characteristic of the aged and diseased valve (112, 132). This lymphocytic infiltrate accompanies increased neovascularization and osseous metaplasia, hallmark histological signs of CAVD (36). In addition, at a transcript level, five of the ten most upregulated pathways in calcified versus noncalcified AVs directly involve T lymphocyte-specific signaling, while nine involve the immune response (57). Functionally, T cell prevalence in the valve is correlated with increased pressure gradient, an echocardiographic measure of aortic stenosis (111), suggesting close ties between AV calcification or stiffening and T cell infiltration. Furthermore, transcriptomic data from CAVD samples show increased granzyme, perforin, CD8, and IFNγ, affirming the presence of T lymphocytes and suggesting an increased activity level (123). IFNγ specifically has been shown to stymie macrophage capacity for calcium reabsorption and osteoclast activity through the receptor activator of nuclear factor-κB (RANK) system (123). This would propose an antigen-independent role for T lymphocytes in the AV, one in which T cells act to increase inflammation by responding to and contributing to the cytokine milieu rather than initiating and expanding an immune response against a specific antigen. As evidence to the contrary, T lymphocytes both in the valve and in circulation are more likely to be clonal in disease (178, 180), suggesting an antigen-specific immunological response. This finding indicates expansion of an antigen-specific T cell repertoire that may or may not be in response to a CAVD-related antigen.
The described T lymphocyte infiltrate of the AV involves both CD8+ T cells [cytotoxic T lymphocytes (CTLs)], and CD4+ T helper (Th) cells, with a tendency toward Th cell dominance (121, 162). Generally, CTLs respond at a single-cell level to kill infected cells or tumor cells, while Th cells coordinate the immune response by providing “help” to other cells. Each of these cell types consists of many subtypes that have not been investigated in CAVD. A further exploration of potential roles for specific T cell subtypes based on cytokine profile and function is described in the next section.
Separately, T lymphocyte counts have been investigated as a potential hematologic biomarker for CAVD. As mentioned above, circulating T cells are more likely to be clonal with worse calcified disease, and this clonality is associated with the CD28null memory phenotype (178). Conversely, an increase in circulating regulatory T cells is observed in CAVD and is decreased with surgical valve replacement (155). These typically anti-inflammatory cells could play a role as a compensatory mechanism to combat immunologically derived calcification. A unique approach to this biomarker question is the platelet-to-lymphocyte ratio, which is increased in more severe CAVD (4), suggesting a role for platelet-derived endothelial activation in CAVD.
Other Lymphocytes in CAVD
Returning to the valve tissue itself, there has been an increasing interest in natural killer T (NKT) cells in CAVD. NKT cells express a semi-invariant T cell receptor, which allows them to respond to a variety of glycolipids when presented by APCs (181, 182). They are known especially for their ability to rapidly release large amounts of cytokines (38, 182); these cytokine secretion capabilities give them a key role in regulating naïve T cell differentiation. They are also known to 1) play a necessary role in immune tolerance and 2) initiate immune responses when given the appropriate secondary signals from APCs (181). NKT cells accumulate both in the valve and in circulation in CAVD, and are associated with increased pressure gradient across the valve (111, 156). In relation to disease pathophysiology, NKT cell depletion has been shown to lead to either amelioration or aggravation of various autoimmune fibrotic diseases (38, 181), and it is just now being understood that NKT cells have subtypes similar to Th cells. It is likely that their presence corresponds to a general inflammatory state in CAVD, but due to this ambiguity it is unclear whether they play a pathophysiological or compensatory role in this particular disease.
Finally, enrichment of B lymphocytes in the valve is associated with increased severity of disease (126). The major role of B lymphocytes is antibody secretion against a specific antigen. Although this antigen has not been identified, the presence of B lymphocytes again highlights the possibility of an antigen-dependent immune response in CAVD.
CYTOKINE PROFILING PROVIDES SPECIFICS ON THE IMMUNE COMPONENT OF CAVD
There is a widespread upregulation of cytokines in the diseased valve, summarized in Table 2. A comprehensive look at transcriptional and protein level changes in CAVD, including cytokines, was done by Schlotter et al. (152) and compared the proteomic signature of CAVD with those of other diseases. Using protein-protein interaction networks, that study found that, in addition to atherosclerosis, hypercholesterolemia, and myocardial infarction, CAVD is also significantly similar to systemic lupus erythematosus, dermatomyositis, vasculitis syndrome, and Takayasu arteritis, all of which involve autoantibodies against specific cellular self-antigens, promoting an adaptive immunity theory. However, further work is needed to understand the commonalities and differences between CAVD and these immune-mediated diseases. To best understand the current data on cytokine enrichment in CAVD, it is useful to group and contextualize them within the immune system.
Table 2.
Cytokines in calcific aortic valve disease
| Cytokine | Immunological Role | Finding | References |
|---|---|---|---|
| Acute phase reactants | |||
| IL-1β | Acute phase reactant | ↑ in CAVD; ↑ in valves with more severe remodeling | (76) |
| IL-1R antagonist | Opposes IL-1 activity | ↓ in AS | (88) |
| IL-6 | Acute phase reactant; promotes T-cell maturation | ↑ in CAVD; ↑ in valves with more severe remodeling; IL6R SNP decreases severity of AS | (45, 170, 184) |
| TNFα | Acute phase reactant | ↑ in CAVD; ↑ with increased inflammation in the valve | (77, 170) |
| Chemokines | |||
| CCL11 | Recruits eosinophils | ↑ in CAVD | (57, 152) |
| CCL19 | Recruits CCR7+ dendritic cells and T cells | ↑ in CAVD | (152) |
| CCL21 | Recruits CCR7+ T cells | ↑ in CAVD | (152) |
| CXCL5 | Recruits angiogenic neutrophils | ↑ in CAVD | (57, 152) |
| CXCL9 | Recruits T cells | ↑ in CAVD | (152) |
| Promoters of specific cell-type maturation | |||
| IFNγ | Acute phase reactant; promotes Th1 maturation | ↑ in CAVD | (123) |
| IL-33 | Promotes Th2 maturation | Present in AS; its receptor, sT2, is increased in plasma in patients with more severe AS, more predictive than BNP for heart failure symptom development, and increased in patients with AS compared with AR | (84, 151) |
| TGF-β | Immunosuppressive; profibrotic; promotes Th17 maturation | ↑ in CAVD | (74) |
| IL-17RA | Receptor for IL-17; promotes Th17 maturation | Nonsignificantly increased in plasma of patients who progress to aortic valve replacement | (98) |
| IL-10 | Immunosuppressive; promotes Treg maturation | Present in CAVD; SNPs in IL10 are associated with CAVD | (8, 175) |
| IL-18 | Promotes T-cell maturation | ↑ in patients with more severe aortic stenosis | (125) |
| M-CSF | Promotes macrophage maturation | Present in CAVD | (175) |
| IL-32 | Proinflammatory | ↑ in CAVD | (171) |
CAVD, calcific aortic valve disease; Th, T helper; IL, interleukin; TNFα, tumor necrosis factor-α; CCL, C-C motif ligand; CXCL, C-X-C motif ligand; CCR, C-C receptor; IFNγ, interferon-γ; TGF-β, transforming growth factor-β; M-CSF, macrophage colony-stimulating factor; SNP, single nucleotide polymorphisms; AS, aortic stenosis; BNP, brain natriuretic peptide; AR, aortic regurgitation.
Acute Phase Reactants
The first significant grouping of cytokines enriched in CAVD is acute phase reactants. Acute phase reactants are general markers of an inflammatory process and serve to activate and recruit an expanded immune response to a tissue withstanding insult, typically infection, but also injury or early neoplastic formation. TNFα, IL-1β, and IL-6, all common acute phase reactants, are increased in CAVD (45, 76, 170). As further evidence of the significance of immune recruitment, single nucleotide polymorphisms (SNPs) in the receptor for IL-6 are associated with decreased severity of aortic stenosis in human disease (184), and IL-1 receptor antagonist is decreased in calcified valves (88). Similarly, in murine models, T cell-specific loss of the IL-1 receptor antagonist leads to apparent thickening of the AV by 12 wk of age (73). This suggests that the T cell response to acute phase reactants plays a role in AV disease states. The data on these cytokines serve as a nonspecific signal that inflammation and leukocyte recruitment are occurring in CAVD, but additional specific cytokine expression data can inform understanding of the types of cells recruited, and what they might be doing in the valve.
Chemokines
Cytokines often serve as recruitment signals and in these situations are termed “chemokines”, as they promote chemotaxis of leukocytes along a molecular gradient and toward the site of insult. Generally speaking, the acute phase reactants listed above also serve as broad immune cell chemokines. Additionally, increased transcription of C-X-C motif ligand 5 (CXCL5; chemokine for neutrophils), CXCL9 (T cells), CCL19 and CCL21 (APCs and naïve T cells), and CCL11 (eosinophils) have been identified in CAVD (57, 152). Each of these cell types may play different roles in the calcification process. Although neutrophils are critical to the initial immune response to injury, they have not often been identified or studied in CAVD. CXCL5, it seems, is more likely part of a general inflammatory process. CXCL9 acts primarily to recruit Th lymphocytes, CTLs, and natural killer cells (56, 87, 173). This increased expression of CXCL9 would thus be associated with a similar phenomenon as the acute phase reactants listed above, wherein its expression connotes a general signal of local inflammation. CCL19 and CCL21 typically recruit CCR7+ dendritic cells and T lymphocytes (60). This brings together antigen-presenting APCs and naïve T lymphocytes to activate the T cells against an antigen and strongly suggests an antigen-related response in CAVD. Finally, CCL11 serves to recruit eosinophils, a small population of granule-filled leukocytes that can be elevated in asthma, allergic reactions, and parasitic infections. Eosinophils have not been identified in the valve and are not increased in circulation in CAVD (154). Thus, the role of CCL11 in the AV is unclear.
Cytokines in the T Lymphocyte Maturation Paradigm
Cytokines can also promote maturation of specific cell types. One such paradigm is in CD4+ T cell subspecification into Th1, Th2, Th17, and regulatory T cells. CD4+ naïve T cells develop into one of these four most common subtypes or others that have been recently described, depending on the cytokine milieu and signals in the environment during maturation. Th1 cells are known for responding to intracellular infections, such as viruses, and promoting an immediate inflammatory response to injury by producing IFNγ and IL-1β and promoting CTL function. This cellular outcome is promoted in CAVD by IFNγ, IL-18, and CXCL9 (123, 125, 152, 193). Th2 cells are associated with an immune response to extracellular pathogens, both bacteria and extracellular forms of viruses, and promote a type 2 immune response including eosinophil, mast cell, and M2 macrophage recruitment, IgE antibody class switching in B cells, and tissue repair (174). These cells are also commonly implicated in allergic conditions, most notably asthma (85). IL-33 is the only pro-Th2 cytokine that has been identified in CAVD (151). Regulatory T cells (Tregs) serve to create a tolerogenic environment in which the immune response is inhibited. Conversely to the previously discussed responses, there is no increase in these cells or cytokines in the diseased valve; however, human association studies have shown that SNPs in IL-10, an immunosuppressive cytokine, are associated with aortic stenosis diagnosis (8), and decreased levels of circulating IL-10 correlate with calcium formation in the valve (170). IL-10 not only induces Treg formation (67), but is produced en masse by Tregs as well as other cell types (120, 150). IL-10 then acts, among other immunosuppressive functions, to inhibit monocyte and macrophage inflammation and cytokine production (41, 50), creating a quiescent microenvironment. Thus, a loss of IL-10 would likely lead to increased activation of the immune response in CAVD. This combination of an increased Th1 cytokine signature and a potentially protective role for IL-10 implies that an immune process likely plays a major role in CAVD.
The final major Th subtype is the Th17 cell, a relatively recent discovery (64). Naïve T cells mature into Th17 cells in the presence of transforming growth factor-β (TGF-β) and IL-6, both of which are present in CAVD (45, 74, 81). Th17 cells then characteristically release IL-17A (i.e., IL-17) in combination with other proinflammatory cytokines and have been implicated in tissue-level inflammation and fibrotic autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and psoriasis (81). In the study of cardiovascular disease, IL-17 has been implicated in both hypertension and atherosclerosis. IL-17 genetic deficiency and both IL-17 and IL-17 receptor A (IL-17RA) antibody blockade attenuate hypertension and end-organ hypertensive sequelae (101, 148). Conversely, systemic IL-17 blockade in atherosclerosis has shown mixed results. Initial studies showed that IL-17 blockade or deficiency decrease plaque size (26, 31, 48, 158). Additional work has shown either no effect or a protective role for IL-17 (21, 40, 100, 168), while in vitro studies of macrophage response to IL-17 stimulation have described a role for IL-17 in differentiation, resolution of inflammation, and plaque stabilization (16, 47, 149, 194). These data suggest a nuanced role for IL-17 in arterial wall physiology and atherosclerosis pathophysiology. As already discussed, IL-6 and TGF-β1 are both elevated in CAVD, but in addition there is an association of IL-17RA concentration in the plasma with worsening disease phenotypes (98). These data taken with the literature on Th17 cells in fibrotic diseases and IL-17 in hypertension and atherosclerosis suggest that they may be involved in CAVD.
It is important to note here that in recent years there has been much more work done on the plasticity and heterogeneity of Th cell subtypes, as well as new subtypes altogether, such as T follicular helper cells (39). The model used here is useful in understanding the general milieu of the tissue in disease states but does not comprehensively describe the roles of specific cells. Future work is necessary to determine what combination of these Th cell populations makes up the CD4+ cellular infiltrate in CAVD and how they contribute to pathophysiology.
CYTOKINES PLAY A MAJOR ROLE IN CELLULAR CALCIFICATION
Cytokines with Procalcification Effects
Separate from pathology-based studies of cytokines in CAVD, there have been many in vitro studies to examine the effects of particular cytokines on the AV resident cells. Although this does not inform our understanding of the leukocyte infiltrate, it may inform how this infiltrate leads to cellular calcification. The most commonly used cytokine to induce AVIC calcification is TGF-β1 (51, 71, 74, 115). This cytokine is often implicated in myofibroblast transition of AVICs and other fibroblasts, but its co-occurrence in Th17 maturation highlights possible coordination of these processes. Similarly, IL-6, the other major driver of Th17 maturation, has been identified in calcified valves, is commonly measured in calcification models, and has been used to induce AVIC calcification (45). Taking the opposite approach, silencing IL-6 has been shown to prevent mineralization (45). To that end, activation of the potential therapeutic target cadherin-11 is known to stimulate IL-6 production by AVICs (30). Cadherin-11 blockade has been shown to abrogate the CAVD phenotype in mice and is associated with decreased expression of IL-6 in AVICs and mice and with the IL-6 receptor subunit GP-130 in AVICs (18, 33). Perhaps the efficacy of this strategy in mice is due to a decreased capability of AVICs to produce and/or respond to IL-6 as a calcification stimulus in interactions with hematopoietic cells.
Additional proinflammatory cytokines have also been shown to promote AVIC calcification. IL-1β treatment of AVICs promotes matrix metalloproteinase expression and remodeling, proliferation, and nuclear factor κB (NF-κB)-driven calcification (76, 122). TNFα, another acute phase reactant, leads to AVIC calcification and promotes calcium absorption by AVICs (77, 187). Finally, IL-18, a Th1-promoting cytokine, leads to myofibroblast transition and NF-κB expression in AVICs (193). These data reinforce the notion that cytokines released by infiltrating leukocytes may lead to AVIC calcification, which is known to be central to CAVD.
Cytokines with Anticalcification Effects
Conversely, IL-37 has been shown to suppress AV lesion formation caused by LPS in mice and calcification in vitro, likely through suppression of Toll-like receptor (TLR) signaling in AVICs (190, 192). IL-37 is generally an immunosuppressive cytokine and has been shown to decrease secretion of inflammatory cytokines in many models (128). TLRs are a mechanism of innate immunity through which cells can recognize general pathogen-associated patterns such as intracellular contents (necrosis), double-stranded RNA (dsRNA, viruses), LPS (bacteria), and glycolipids (bacteria) (5), and are expressed widely in many tissues and cell types, including AVICs (114). Interestingly, TLR signaling has been identified as a mechanism for AVIC calcification in response to hypercholesterolemic states, tissue injury, or potentially infectious etiologies (10, 49, 53, 109). In addition, TLR activation stimulates cytokine expression (188). Thus, it is quite plausible that TLR activation of AVICs and physiologically present APCs is an instigating event of leukocyte recruitment. In fact, LPS and dsRNA activation of TLRs leads to AVIC secretion of IL-6 and IL-8, further highlighting this role of AVICs as both a responder to and an instigator of the immune response (114, 189, 191). Elsewhere in the process, the increased expression of TLRs on leukocytes compared with other cell types highlights the potential role of TLRs in promoting a cellular immune process (118, 188). The capability of AVICs to both secrete and respond to the general cytokine milieu emphasizes the role that immune cells can play in this disease.
IMMUNE CELLS IN CARDIOVASCULAR DISEASE
Macrophages in Cardiovascular Disease
Although it is yet to be well understood how leukocytes impact CAVD, much can be learned from the role of these cells in other cardiovascular diseases. It has long been known that macrophages play a central role in atherosclerosis (22), although only recently has this understanding grown to incorporate an adaptive immune response to self-antigens (63). It is now understood that a Th1 response is proatherogenic, whereas a Treg response inhibits atherogenesis (2, 63), and that MHC II presentation of extracellular antigens by dendritic cells is necessary to drive this CD4+ T cell response (147). This highlights the role of the adaptive immune response in atherosclerosis. Similarly, it is now known that T lymphocytes are necessary for angiotensin II-dependent hypertension, the most significant risk factor for CAVD (59, 66, 96). In response to known hypertension stimuli, reactive oxygen species accumulate in dendritic cells and promote formation of immunogenic molecules, which are presented to T cells, leading to cytokine secretion and the hypertensive phenotype (15, 80). The role of inflammation in atherosclerosis and hypertension is well established and has been the focus of multiple clinical trials (143–145).
It should be noted that, although atherosclerosis and hypertension are common comorbidities and risk factors for CAVD, trials attempting to treat CAVD with lipid-lowering therapy have shown little efficacy (37, 146), and trials with antihypertensive agents have decreased left ventricle remodeling but had little effect on valve function (24, 96, 107). This would suggest that CAVD has a different pathophysiology or etiology than these two diseases. However, management of systolic hypertension has been shown to improve progression rates, and elevated lipoprotein(a) is associated with worse rates of progression (24, 28, 29). The relationship between these three diseases and their etiologies is still unclear; nonetheless, principles from the study of hyperlipidemia and hypertension can be used to guide future investigation in CAVD.
Macrophages play a role in the pathophysiology of both the vasculature and the heart itself (11, 165). It has been found that macrophages play a significant role in cardiac development (89) and facilitate electrical conduction (69). Additionally, there is a diversity of macrophages in the heart (11, 46), and a bifurcation of macrophage populations after myocardial infarction (124), some of which limit adverse effects after infarction (42). These data suggest that macrophages likely play a significant role in both pathophysiology and healing in diseases of the myocardium.
T Lymphocytes in Cardiovascular Disease
T lymphocytes also play a role in cardiovascular disease. T cells promote pathological remodeling in myocardial infarction in coordination with macrophages (14) and play a major role in myocarditis. Although there are many subtypes of myocarditis, an increasing area of interest is myocarditis caused by immune checkpoint blockade in cancer treatment. Immune checkpoint blockade involves blocking one of a number of inhibitory signals for T cells, the clinically used and most commonly known being cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein-1 (PD-1), which are crucial for negative regulation of T cell activity (169). This blockade increases T cell activation, allowing for killing of cancer cells by the body’s own adaptive immune system. Unfortunately, mice with PD-1 deficiency develop dilated cardiomyopathy and myocarditis (127, 169), which are only now starting to be seen in human patients taking the new therapies (75). This suggests that when lacking the appropriate suppressive mechanisms there is a T cell tropism for cardiac tissue. This may be an antigen-specific response and has been identified in part to be in response to cardiac troponin I, a ubiquitously expressed protein in cardiomyocytes (131). These findings highlight the ability for T lymphocytes to respond to cardiac antigens, many of which are shared across the myocardium and valve tissue.
Separately, ubiquitous overexpression of IL-23, a proinflammatory cytokine that induces Th17 differentiation, in a mouse model leads to an accumulation of IL-17-producing T lymphocytes in the AV (142). Although this is not a study of CAVD, this finding suggests a tendency in inflammatory states for proinflammatory T lymphocytes to accumulate in the AV in chronic inflammatory states, echoing the cardiac tropism seen in PD-1-deficient mice. Finally, macrophages and monocytes play a key role in rheumatic valve fibrosis (113). Although this disease model is not the same as CAVD, some insight can be taken from the way that macrophages direct this process of valvular fibrosis. Overall, it is clear that the adaptive immune system 1) plays a crucial role in many cardiovascular diseases and 2) has a tropism for the heart and valves. It remains to be seen how this evidence can inform the incorporation of the immune system into CAVD models.
POTENTIAL SELF-ANTIGENS IN CAVD
It is not yet understood how the immune system directly or indirectly impacts AV calcification in vivo; however, a general paradigm can be proposed based on our knowledge of the immune system and its implication in other cardiovascular diseases (Fig. 1). The first relevant step in adaptive immunity is antigen recognition. Here, we discuss three potential avenues for antigen-dependent activation of the adaptive immune system: infectious agents, cellular death, and oxidant species.
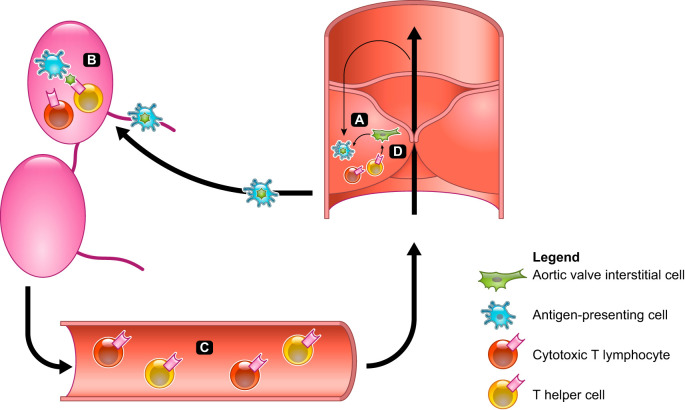
Fig. 1.
Model for adaptive immune system involvement. A: chronic inflammatory conditions, immunological aging, and mechanical stimuli promote cell death and cytokine secretion by aortic valve interstitial cells (green) and antigen-presenting cells (APCs; blue), leading to activation of local APCs. B: APCs follow the lymphatic drainage to lymph nodes, where they prime and activate antigen-specific cytotoxic T lymphocytes and T helper cells (red and yellow, respectively) against valvular antigens. C: activated T cells enter the circulation and are recruited to the valve by local chemokine expression from both APCs and fibroblasts. D: T cells that have entered the valve recognize local antigens and release inflammatory cytokines, recruiting further immune cells and leading to increased myofibroblast transition of aortic valve interstitial cells.
Infectious Agents
Although not identified in CAVD, viral infection, specifically with cytomegalovirus (CMV), has been suggested as a causative factor in age-related diseases (106, 134). For immune competent individuals, CMV is easily dispensed with by the immune system, but its prevalence is incredibly high in the United States and progresses with age, with 36.3% of 6- to 11-yr-olds and 90.8% of >80-yr-olds having had the disease at some point and developed antibodies (161). Although CMV typically has a self-limited acute clinical phase, it has been shown to cause immune dysregulation throughout the aging process, even leading to increased risk of cardiovascular disease (1, 72, 139, 160). Past CMV infection leads to increased oligoclonality of T cell receptors, implying expansion of a subset of T cells targeting a specific antigen or related antigens, and a higher proportion of “T memory cells with naïve phenotype”, which can rapidly release cytokines and have high cytotoxic potential (1, 139). It has been suggested that these oligoclonal T cells may recognize self-antigens in combination with viral particles or self-antigens that resemble viral particles. This leads to fibrotic autoimmune reactions in response to new stimuli like medications (135), but it could also prime the valve for a fibrotic response. In regard to bacteria, although LPS derived from gram-negative bacterial cell walls is often used to stimulate TLRs in in vitro calcification experiments, neither gram-negative nor the more common gram-positive bacterial endocarditis has been shown to be associated with CAVD.
Cell Death
Cell death also provides antigens that may be recognized by the immune system. The immune system has a common mechanism for recognizing “self” cells, through which resident cells present molecules from their cytoplasm to T cells on major histocompatibility complex I. Through the T cell education process, the immune system learns not to react to these presentations. However, apoptotic cells can release self-proteins that do not typically exist in the cytoplasmic compartment and therefore are not typically presented to T cells (6). These molecules can then trigger a CTL response. It has long been known that TGF-β1 induces apoptosis (25, 34, 74, 130, 153), and it is found in CAVD and commonly used in in vitro models of AV calcification, as discussed in previous sections. In addition, the unique mechanical stimuli in the valve exacerbate this pathway and provoke cellular apoptosis (51). Finally, it is also reported that there is increased cell death and autophagy in CAVD (159), which could lead to increased recycled cellular products in the extracellular space. In concert, these findings suggest heightened cell death in the valve, which may cause a release of self-antigens recognizable by the immune system.
Reactive Oxygen Species
Finally, it is possible that the native APCs in the AV respond to similar stimuli as in hypertension and atherosclerosis. As discussed above, dendritic cells respond to hypertensive stimuli by increasing production of reactive oxygen species, which then transform intracellular molecules into neo-antigens. These are recognized as pathogens by the immune system, leading to a systemic immune response that promotes the hypertensive response (15, 80). A similar system has been proposed in atherosclerosis, wherein macrophages present modified lipoproteins, necrotic debris, or other altered self-structures (103, 136). Since hyperlipidemia and hypertension are two major risk factors and common comorbidities for CAVD, it is plausible that this process is occurring in the early calcified valve (96). In fact, there is an increase in reactive oxygen species in early valve disease in both mice and humans (94, 116, 177). This increase is focused around sites of calcification, comes before hemodynamic changes, and does not increase after the initiation of the disease phenotype (94, 117). This finding, increased prevalence and a dynamic range early in the disease process, advocates for a role of reactive oxygen species in the pathogenesis of CAVD similar to that found in hypertension. Although these species have a clear impact in lipid metabolism and AV cell calcification (19, 20, 110, 116), their role in initiating an adaptive immune response in CAVD is yet unknown.
CHRONIC INFLAMMATION AND OTHER SECONDARY STIMULI IN CAVD PATIENTS
In addition to the stimulating antigen, immune system responses can be dictated or directed by the microenvironment. These include factors secreted by cells in the area and mechanical cues. The most obvious factor that could play a role here is the immune activity of AV resident cells. In response to inflammatory stimuli, AVICs secrete IL-6, IL-8 (10, 45, 189), and TGF-β1 (74). More broadly, it is known that many fibroblast-like cells are capable of secreting immune modulating factors that directly impact immune cell function in both physiological and diseased states (23, 82, 83, 102). In CAVD, the tendency of AVICs to secrete both IL-6 and TGF-β1, directors of the Th17 cell fate (81), and the presence of these cytokines in the AV in ex vivo studies suggest that naïve T cells may be more likely to develop into Th17 cells. Given their role in tissue inflammation, this development may play a role in CAVD.
Another factor at play is the chronic inflammatory state that defines CAVD risk factors such as hypertension, diabetes, and hyperlipidemia. Hypertensive patients have increased circulating levels of acute phase reactants TNFα and IL-6 (17), and IL-17A is necessary for sustained hypertension in mice (101, 148), highlighting the presence of an inflammatory state in hypertension. Similarly, diabetes is defined by an IL-1-dependent increase in acute phase reactants (43, 86), and atherosclerosis is associated with an increase in inflammatory markers (62, 97). Each of these diseases leads to inflammation in the vascular wall, and this baseline inflammation could make the AV a prime location for immune activation.
The final potential stimulus to discuss here is mechanical forces, which have previously been implicated in the vascular inflammation of hypertension (99). These forces are at their highest in the dynamic and high-pressure setting of the AV, a phenomenon reviewed well by Balachandran et al. (12). Whereas mechanical forces have been used consistently to examine AVIC calcification, their role in immune cell maturation and function is not well understood. Although ill defined, mechanical forces clearly play a role in leukocyte physiology (70). Strain-related studies of both dendritic cells and macrophages show increased expression of inflammatory molecules, but definitive phenotypes are inconclusive (13, 91, 138, 176, 185). Nonetheless, it is clear that coupling the known mechanical environment of the AV with the relatively new appreciation of the potential role of the hematopoietic cells contained within shows promise in revealing novel pathogenic pathways in CAVD.
CONCLUSIONS AND FUTURE DIRECTIONS
A general paradigm can be proposed, by which APCs in the healthy AV initiate an adaptive immune response contributing to end-stage CAVD. Namely, they may initially present either a neo-antigen derived from chronic inflammatory states and/or cell death or an antigen that is newly recognized by malfunctioning T cells. Responding to the cytokine milieu in the valve, these APCs give a secondary signal to activate T cells into proinflammatory phenotypes. Additionally, the cytokine milieu directing immune cell recruitment and maturation is likely affected by the aforementioned chronic inflammatory states, a unique mechanical environment, and the cytokine expression of AVICs. The activated T cells then home in to the valve, where they respond and contribute to the cytokine milieu. As a result of the microenvironment created, AVICs undergo the TGF-β-induced apoptosis pathway and begin the calcification process. Although there are steps in this proposed pathophysiology that are yet to be described or interrogated, it is also clear at this time that integrating the adaptive immune system into future research and disease models is necessary for a more complete explanation of this disease and as a basis for future drug discovery for this prevalent and deadly disease.
GRANTS
We acknowledge funding from National Institutes of Health Grants R35-HL135970, K08-HL-121671, DP2-HL-137166, and T32-GM-007347 and Fondation Leducq.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.R. prepared figures; M.A.R. drafted manuscript; M.S.M. and W.D.M. edited and revised manuscript; M.A.R., M.S.M., and W.D.M. approved final version of manuscript.
REFERENCES
- 1.Aiello AE, Chiu YL, Frasca D. How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? Geroscience 39: 261–271, 2017. doi: 10.1007/s11357-017-9983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12: 178–180, 2006. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 3.Akahori H, Tsujino T, Naito Y, Yoshida C, Lee-Kawabata M, Ohyanagi M, Mitsuno M, Miyamoto Y, Daimon T, Masuyama T. Intraleaflet haemorrhage as a mechanism of rapid progression of stenosis in bicuspid aortic valve. Int J Cardiol 167: 514–518, 2013. doi: 10.1016/j.ijcard.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 4.Akdag S, Akyol A, Asker M, Duz R, Gumrukcuoglu HA. Platelet-to-lymphocyte ratio may predict the severity of calcific aortic stenosis. Med Sci Monit 21: 3395–3400, 2015. doi: 10.12659/MSM.894774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 4: 499–511, 2004. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 6.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392: 86–89, 1998. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 7.Alexander KA, Chang MK, Maylin ER, Kohler T, Müller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, Pettit AR. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 26: 1517–1532, 2011. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 8.An Y, Wang YT, Ma YT, Wulasihan M, Huang Y, Adi D, Yang YN, Ma X, Li XM, Xie X, Huang D, Liu F, Chen BD. IL-10 genetic polymorphisms were associated with valvular calcification in Han, Uygur and Kazak populations in Xinjiang, China. PLoS One 10: e0128965, 2015. doi: 10.1371/journal.pone.0128965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anstine LJ, Horne TE, Horwitz EM, Lincoln J. Contribution of extra-cardiac cells in murine heart valves is age-dependent. J Am Heart Assoc 6: 1–13, 2017. doi: 10.1161/JAHA.117.007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babu AN, Meng X, Zou N, Yang X, Wang M, Song Y, Cleveland JC, Weyant M, Banerjee A, Fullerton DA. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg 86: 71–76, 2008. doi: 10.1016/j.athoracsur.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 24: 1234–1245, 2018. doi: 10.1038/s41591-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balachandran K, Sucosky P, Yoganathan AP. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflamm 2011: 263870, 2011. doi: 10.4061/2011/263870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballotta V, Driessen-Mol A, Bouten CVC, Baaijens FP. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials 35: 4919–4928, 2014. doi: 10.1016/j.biomaterials.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD, Activated T. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail 10: e003688, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep 21: 1009–1020, 2017. doi: 10.1016/j.celrep.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barin JG, Baldeviano GC, Talor MV, Wu L, Ong S, Quader F, Chen P, Zheng D, Caturegli P, Rose NR, Ciháková D. Macrophages participate in IL-17-mediated inflammation. Eur J Immunol 42: 726–736, 2012. doi: 10.1002/eji.201141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J Hum Hypertens 19: 149–154, 2005. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 18.Bowler MA, Bersi MR, Ryzhova LM, Jerrell RJ, Parekh A, Merryman WD. Cadherin-11 as a regulator of valve myofibroblast mechanobiology. Am J Physiol Heart Circ Physiol 315: H1614–H1626, 2018. doi: 10.1152/ajpheart.00277.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowler MA, Merryman WD. In vitro models of aortic valve calcification: solidifying a system. Cardiovasc Pathol 24: 1–10, 2015. doi: 10.1016/j.carpath.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, Ferrari G. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol 33: e66–e74, 2013. doi: 10.1161/ATVBAHA.112.300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brauner S, Jiang X, Thorlacius GE, Lundberg AM, Östberg T, Yan ZQ, Kuchroo VK, Hansson GK, Wahren-Herlenius M. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc Res 114: 158–167, 2018. doi: 10.1093/cvr/cvx181. [DOI] [PubMed] [Google Scholar]
- 22.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem 52: 223–261, 1983. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 23.Bucala R, Ritchlin C, Winchester R, Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med 173: 569–574, 1991. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bull S, Loudon M, Francis JM, Joseph J, Gerry S, Karamitsos TD, Prendergast BD, Banning AP, Neubauer S, Myerson SG. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging 16: 834–841, 2015. doi: 10.1093/ehjci/jev043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bursch W, Oberhammer F, Jirtle RL, Askari M, Sedivy R, Grasl-Kraupp B, Purchio AF, Schulte-Hermann R. Transforming growth factor-beta 1 as a signal for induction of cell death by apoptosis. Br J Cancer 67: 531–536, 1993. doi: 10.1038/bjc.1993.98. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res 110: 675–687, 2012. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calin MV, Manduteanu I, Dragomir E, Dragan E, Nicolae M, Gan AM, Simionescu M. Effect of depletion of monocytes/macrophages on early aortic valve lesion in experimental hyperlipidemia. Cell Tissue Res 336: 237–248, 2009. doi: 10.1007/s00441-009-0765-2. [DOI] [PubMed] [Google Scholar]
- 28.Capoulade R, Chan KL, Yeang C, Mathieu P, Bossé Y, Dumesnil JG, Tam JW, Teo KK, Mahmut A, Yang X, Witztum JL, Arsenault BJ, Després JP, Pibarot P, Tsimikas S. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol 66: 1236–1246, 2015. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Capoulade R, Clavel MA, Mathieu P, Côté N, Dumesnil JG, Arsenault M, Bédard E, Pibarot P. Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur J Clin Invest 43: 1262–1272, 2013. doi: 10.1111/eci.12169. [DOI] [PubMed] [Google Scholar]
- 30.Chang SK, Noss EH, Chen M, Gu Z, Townsend K, Grenha R, Leon L, Lee SY, Lee DM, Brenner MB. Cadherin-11 regulates fibroblast inflammation. Proc Natl Acad Sci USA 108: 8402–8407, 2011. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, Yan XX, Nie SF, Liao MY, Cheng Y, Mallat Z, Liao YH. Inhibition of IL-17A in atherosclerosis. Atherosclerosis 215: 471–474, 2011. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med 206: 497–505, 2009. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark CR, Bowler MA, Snider JC, Merryman WD, David Merryman W. Targeting cadherin-11 prevents Notch1-mediated calcific aortic valve disease. Circulation 135: 2448–2450, 2017. doi: 10.1161/CIRCULATIONAHA.117.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER 3rd, Gorman JH 3rd, Gorman RC, Levy RJ. Transforming growth factor-β1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg 83: 946–953, 2007. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol 63, 25 Pt A: 2852–2861, 2014. doi: 10.1016/j.jacc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Coté N, Mahmut A, Bosse Y, Couture C, Pagé S, Trahan S, Boulanger MC, Fournier D, Pibarot P, Mathieu P. Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation 36: 573–581, 2013. doi: 10.1007/s10753-012-9579-6. [DOI] [PubMed] [Google Scholar]
- 37.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators . A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 352: 2389–2397, 2005. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 38.Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol 18: 559–574, 2018. doi: 10.1038/s41577-018-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 41: 529–542, 2014. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, Tsutsui H, Uede T. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 32: 273–280, 2012. doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 41.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174: 1209–1220, 1991. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, Wong A, Zaman R, Barbu I, Besla R, Lavine KJ, Razani B, Ginhoux F, Husain M, Cybulsky MI, Robbins CS, Epelman S. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 20: 29–39, 2019. doi: 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98–107, 2011. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 44.Egan KP, Kim JH, Mohler ER III, Pignolo RJ. Role for circulating osteogenic precursor cells in aortic valvular disease. Arterioscler Thromb Vasc Biol 31: 2965–2971, 2011. doi: 10.1161/ATVBAHA.111.234724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Husseini D, Boulanger M-CC, Mahmut A, Bouchareb R, Laflamme M-HH, Fournier D, Pibarot P, Bossé Y, Mathieu P. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: implication for calcific aortic valve disease. J Mol Cell Cardiol 72: 146–156, 2014. doi: 10.1016/j.yjmcc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40: 91–104, 2014. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erbel C, Akhavanpoor M, Okuyucu D, Wangler S, Dietz A, Zhao L, Stellos K, Little KM, Lasitschka F, Doesch A, Hakimi M, Dengler TJ, Giese T, Blessing E, Katus HA, Gleissner CA. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol 193: 4344–4355, 2014. doi: 10.4049/jimmunol.1400181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol 183: 8167–8175, 2009. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 49.Fernández-Pisonero I, López J, Onecha E, Dueñas AI, Maeso P, Crespo MS, San Román JA, García-Rodríguez C. Synergy between sphingosine 1-phosphate and lipopolysaccharide signaling promotes an inflammatory, angiogenic and osteogenic response in human aortic valve interstitial cells. PLoS One 9: e109081, 2014. doi: 10.1371/journal.pone.0109081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol 147: 3815–3822, 1991. . [PubMed] [Google Scholar]
- 51.Fisher CI, Chen J, Merryman WD. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech Model Mechanobiol 12: 5–17, 2013. doi: 10.1007/s10237-012-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujisaka T, Hoshiga M, Hotchi J, Takeda Y, Jin D, Takai S, Hanafusa T, Ishizaka N. Angiotensin II promotes aortic valve thickening independent of elevated blood pressure in apolipoprotein-E deficient mice. Atherosclerosis 226: 82–87, 2013. doi: 10.1016/j.atherosclerosis.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 53.García-Rodríguez C, Parra-Izquierdo I, Castaños-Mollor I, López J, San Román JA, Sánchez Crespo M. Toll-like receptors, inflammation, and calcific aortic valve disease. Front Physiol 9: 201, 2018. doi: 10.3389/fphys.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol 13: 368–380, 2017. doi: 10.1038/nrneph.2017.51. [DOI] [PubMed] [Google Scholar]
- 55.Gössl M, Khosla S, Zhang X, Higano N, Jordan KL, Loeffler D, Enriquez-Sarano M, Lennon RJ, McGregor U, Lerman LO, Lerman A. Role of circulating osteogenic progenitor cells in calcific aortic stenosis. J Am Coll Cardiol 60: 1945–1953, 2012. doi: 10.1016/j.jacc.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32: 659–702, 2014. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 57.Guauque-Olarte S, Droit A, Tremblay-Marchand J, Gaudreault N, Kalavrouziotis D, Dagenais F, Seidman JG, Body SC, Pibarot P, Mathieu P, Bossé Y. RNA expression profile of calcified bicuspid, tricuspid, and normal human aortic valves by RNA sequencing. Physiol Genomics 48: 749–761, 2016. doi: 10.1152/physiolgenomics.00041.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunawardene P, Bermeo S, Vidal C, Al-Saedi A, Chung P, Boersma D, Phu S, Pokorski I, Suriyaarachchi P, Demontiero O, Duque G. Association between circulating osteogenic progenitor cells and disability and frailty in older persons: The nepean osteoporosis and frailty study. J Gerontol A Biol Sci Med Sci 71: 1124–1130, 2016. doi: 10.1093/gerona/glv190. [DOI] [PubMed] [Google Scholar]
- 59.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haessler U, Pisano M, Wu M, Swartz MA. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc Natl Acad Sci USA 108: 5614–5619, 2011. doi: 10.1073/pnas.1014920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajdu Z, Romeo SJ, Fleming PA, Markwald RR, Visconti RP, Drake CJ. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol 51: 955–965, 2011. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352: 1685–1695, 2005. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 63.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 12: 204–212, 2011. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 64.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol 18: 349–356, 2006. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 65.Helske S, Lindstedt KA, Laine M, Mäyränpää M, Werkkala K, Lommi J, Turto H, Kupari M, Kovanen PT. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol 44: 1859–1866, 2004. doi: 10.1016/j.jacc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 66.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296: R208–R216, 2009. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M, Wong M, Fuller SJ, Nanan R. IL-10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J Immunol 195: 3665–3674, 2015. doi: 10.4049/jimmunol.1402898. [DOI] [PubMed] [Google Scholar]
- 68.Hulin A, Anstine LJ, Kim AJ, Potter SJ, DeFalco T, Lincoln J, Yutzey KE. Macrophage transitions in heart valve development and myxomatous valve disease. Arterioscler Thromb Vasc Biol 38: 636–644, 2018. doi: 10.1161/ATVBAHA.117.310667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M. Macrophages facilitate electrical conduction in the heart. Cell 169: 510–522.e20, 2017. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huse M. Mechanical forces in the immune system. Nat Rev Immunol 17: 679–690, 2017. doi: 10.1038/nri.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hutcheson JD, Ryzhova LM, Setola V, Merryman WD. 5-HT(2B) antagonism arrests non-canonical TGF-β1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol 53: 707–714, 2012. doi: 10.1016/j.yjmcc.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isobe KI, Nishio N, Hasegawa T. Immunological aspects of age-related diseases. World J Biol Chem 8: 129–137, 2017. doi: 10.4331/wjbc.v8.i2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Isoda K, Matsuki T, Kondo H, Iwakura Y, Ohsuzu F. Deficiency of interleukin-1 receptor antagonist induces aortic valve disease in BALB/c mice. Arterioscler Thromb Vasc Biol 30: 708–715, 2010. doi: 10.1161/ATVBAHA.109.201749. [DOI] [PubMed] [Google Scholar]
- 74.Jian B, Narula N, Li QY, Mohler ER III, Levy RJ. Progression of aortic valve stenosis: TGF-β1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 75: 457–465, 2003. doi: 10.1016/S0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 75.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375: 1749–1755, 2016. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kiliç R, Sarikoç A, Brueckmann M, Vahl C, Hagl S, Haase KK, Borggrefe M. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 170: 205–211, 2003. doi: 10.1016/S0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 77.Kaden JJ, Kiliç R, Sarikoç A, Hagl S, Lang S, Hoffmann U, Brueckmann M, Borggrefe M. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int J Mol Med 16: 869–872, 2005. doi: 10.3892/ijmm.16.5.869. . [DOI] [PubMed] [Google Scholar]
- 78.Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, Kessler JA. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells 27: 150–156, 2009. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katayama S, Umetani N, Hisada T, Sugiura S. Bicuspid aortic valves undergo excessive strain during opening: a simulation study. J Thorac Cardiovasc Surg 145: 1570–1576, 2013. doi: 10.1016/j.jtcvs.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 80.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 27: 485–517, 2009. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 82.Kuen J, Darowski D, Kluge T, Majety M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS One 12: e0182039, 2017. doi: 10.1371/journal.pone.0182039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Låg M, Rodionov D, Øvrevik J, Bakke O, Schwarze PE, Refsnes M. Cadmium-induced inflammatory responses in cells relevant for lung toxicity: Expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicol Lett 193: 252–260, 2010. doi: 10.1016/j.toxlet.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 84.Lancellotti P, Dulgheru R, Magne J, Henri C, Servais L, Bouznad N, Ancion A, Martinez C, Davin L, Le Goff C, Nchimi A, Piérard L, Oury C. Elevated plasma soluble sT2 is associated with heart failure symptoms and outcome in aortic stenosis. PLoS One 10: e0138940, 2015. doi: 10.1371/journal.pone.0138940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 111: 450–463, 2003. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 86.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356: 1517–1526, 2007. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 87.Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML, Clausen BE, De Jong EC. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol 83: 525–535, 2005. doi: 10.1111/j.1440-1711.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 88.Lee JH, Meng X, Weyant MJ, Reece TB, Cleveland JC Jr, Fullerton DA. Stenotic aortic valves have dysfunctional mechanisms of anti-inflammation: implications for aortic stenosis. J Thorac Cardiovasc Surg 141: 481–486, 2011. doi: 10.1016/j.jtcvs.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res 118: 1498–1511, 2016. doi: 10.1161/CIRCRESAHA.115.308270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levy RJ, Schoen FJ, Howard SL. Mechanism of calcification of porcine bioprosthetic aortic valve cusps: role of T-lymphocytes. Am J Cardiol 52: 629–631, 1983. doi: 10.1016/0002-9149(83)90040-1. [DOI] [PubMed] [Google Scholar]
- 91.Lewis JS, Dolgova NV, Chancellor TJ, Acharya AP, Karpiak JV, Lele TP, Keselowsky BG. The effect of cyclic mechanical strain on activation of dendritic cells cultured on adhesive substrates. Biomaterials 34: 9063–9070, 2013. doi: 10.1016/j.biomaterials.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li G, Qiao W, Zhang W, Li F, Shi J, Dong N. The shift of macrophages toward M1 phenotype promotes aortic valvular calcification. J Thorac Cardiovasc Surg 153: 1318–1327.e1, 2017. doi: 10.1016/j.jtcvs.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 93.Li XF, Wang Y, Zheng DD, Xu HX, Wang T, Pan M, Shi JH, Zhu JH. M1 macrophages promote aortic valve calcification mediated by microRNA-214/TWIST1 pathway in valvular interstitial cells. Am J Transl Res 8: 5773–5783, 2016. [PMC free article] [PubMed] [Google Scholar]
- 94.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, Laurindo FR. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 28: 463–470, 2008. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 95.Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn 230: 239–250, 2004. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- 96.Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P. Calcific aortic stenosis. Nat Rev Dis Primers 2: 16006, 2016. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord 27, Suppl 3: S35–S40, 2003. doi: 10.1038/sj.ijo.0802498. [DOI] [PubMed] [Google Scholar]
- 98.Ljungberg J, Janiec M, Bergdahl IA, Holmgren A, Hultdin J, Johansson B, Näslund U, Siegbahn A, Fall T, Söderberg S. Proteomic biomarkers for incident aortic stenosis requiring valvular replacement. Circulation 138: 590–599, 2018. doi: 10.1161/CIRCULATIONAHA.117.030414. [DOI] [PubMed] [Google Scholar]
- 99.Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, Elijovich F, Laffer CL, Gnecco JS, Noonan J, Maffia P, Jasiewicz-Honkisz B, Czesnikiewicz-Guzik M, Mikolajczyk T, Sliwa T, Dikalov S, Weyand CM, Guzik TJ, Harrison DG. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res 114: 1547–1563, 2018. doi: 10.1093/cvr/cvy112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol 31: 1565–1572, 2011. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Majety M, Pradel LP, Gies M, Ries CH. Fibroblasts influence survival and therapeutic response in a 3D co-culture model. PLoS One 10: e0127948, 2015. doi: 10.1371/journal.pone.0127948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive T cell immunity in atherosclerosis. J Lipid Res 50, Suppl: S364–S369, 2009. doi: 10.1194/jlr.R800092-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity 23: 344–346, 2005. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 105.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 106.Margolis L. Immunoactivation at the crossroads of human disease. Am J Med 128: 562–566, 2015. doi: 10.1016/j.amjmed.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marquis-Gravel G, Redfors B, Leon MB, Généreux P. Medical treatment of aortic stenosis. Circulation 134: 1766–1784, 2016. doi: 10.1161/CIRCULATIONAHA.116.023997. [DOI] [PubMed] [Google Scholar]
- 108.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13, 2014. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mathieu P, Bouchareb R, Boulanger MC. Innate and adaptive immunity in calcific aortic valve disease. J Immunol Res 2015: 851945, 2015. doi: 10.1155/2015/851945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mathieu P, Boulanger MC. Basic mechanisms of calcific aortic valve disease. Can J Cardiol 30: 982–993, 2014. doi: 10.1016/j.cjca.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 111.Mazur P, Mielimonka A, Natorska J, Wypasek E, Gawęda B, Sobczyk D, Kapusta P, Malinowski KP, Kapelak B. Lymphocyte and monocyte subpopulations in severe aortic stenosis at the time of surgical intervention. Cardiovasc Pathol 35: 1–7, 2018. doi: 10.1016/j.carpath.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 112.Mazzone A, Epistolato MC, De Caterina R, Storti S, Vittorini S, Sbrana S, Gianetti J, Bevilacqua S, Glauber M, Biagini A, Tanganelli P. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J Am Coll Cardiol 43: 1670–1676, 2004. doi: 10.1016/j.jacc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 113.Meier LA, Auger JL, Engelson BJ, Cowan HM, Breed ER, Gonzalez-Torres MI, Boyer JD, Verma M, Marath A, Binstadt BA. CD301b/MGL2+ mononuclear phagocytes orchestrate autoimmune cardiac valve inflammation and fibrosis. Circulation 137: 2478–2493, 2018. doi: 10.1161/CIRCULATIONAHA.117.033144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, Weyant MJ, Dinarello CA, Cleveland JC Jr, Fullerton DA. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol 294: C29–C35, 2008. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 115.Merryman WD, Lukoff HD, Long RA, Engelmayr GC Jr, Hopkins RA, Sacks MS. Synergistic effects of cyclic tension and transforming growth factor-β1 on the aortic valve myofibroblast. Cardiovasc Pathol 16: 268–276, 2007. doi: 10.1016/j.carpath.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 52: 843–850, 2008. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller JD, Weiss RM, Serrano KM, Brooks RM 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation 119: 2693–2701, 2009. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol 11: 807–822, 2011. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 119.Milutinovic A, Petrovič D, Zorc M, Vraspir Porenta O, Arko M, Pleskovič A, Alibegovic A, Zorc-Pleskovic R, Milutinovic A. Mast cells might have a protective role against the development of calcification and hyalinisation in severe aortic valve stenosis. Folia Biol (Praha) 62: 160–166, 2016. . [PubMed] [Google Scholar]
- 120.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765, 2001. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 121.Mosch J, Gleissner CA, Body S, Aikawa E. Histopathological assessment of calcification and inflammation of calcific aortic valves from patients with and without diabetes mellitus. Histol Histopathol 32: 293–306, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nadlonek N, Lee JH, Reece TB, Weyant MJ, Cleveland JC Jr, Meng X, Fullerton DA. Interleukin-1 Beta induces an inflammatory phenotype in human aortic valve interstitial cells through nuclear factor kappa Beta. Ann Thorac Surg 96: 155–162, 2013. doi: 10.1016/j.athoracsur.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nagy E, Lei Y, Martínez-Martínez E, Body SC, Schlotter F, Creager M, Assmann A, Khabbaz K, Libby P, Hansson GK, Aikawa E. Interferon-γ released by activated CD8+ T lymphocytes impairs the calcium resorption potential of osteoclasts in calcified human aortic valves. Am J Pathol 187: 1413–1425, 2017. doi: 10.1016/j.ajpath.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Naito Y, Tsujino T, Wakabayashi K, Matsumoto M, Ohyanagi M, Mitsuno M, Miyamoto Y, Hao H, Hirota S, Okamura H, Masuyama T. Increased interleukin-18 expression in nonrheumatic aortic valve stenosis. Int J Cardiol 144: 260–263, 2010. doi: 10.1016/j.ijcard.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 126.Natorska J, Marek G, Sadowski J, Undas A. Presence of B cells within aortic valves in patients with aortic stenosis: relation to severity of the disease. J Cardiol 67: 80–85, 2016. doi: 10.1016/j.jjcc.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 127.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291: 319–322, 2001. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 128.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 11: 1014–1022, 2010. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med 215: 21–33, 2018. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oberhammer F, Bursch W, Tiefenbacher R, Fröschl G, Pavelka M, Purchio T, Schulte-Hermann R. Apoptosis is induced by transforming growth factor-beta 1 within 5 hours in regressing liver without significant fragmentation of the DNA. Hepatology 18: 1238–1246, 1993. doi: 10.1002/hep.1840180533. [DOI] [PubMed] [Google Scholar]
- 131.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 9: 1477–1483, 2003. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 132.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Rydén L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol 23: 1162–1170, 1994. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 133.Padang R, Bagnall RD, Tsoutsman T, Bannon PG, Semsarian C. Comparative transcriptome profiling in human bicuspid aortic valve disease using RNA sequencing. Physiol Genomics 47: 75–87, 2015. doi: 10.1152/physiolgenomics.00115.2014. [DOI] [PubMed] [Google Scholar]
- 134.Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt PW, Deeks SG, Hodis HN, Kaplan RC. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis 205: 1788–1796, 2012. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pavlos R, White KD, Wanjalla C, Mallal SA, Phillips EJ. Severe Delayed Drug Reactions: Role of Genetics and Viral Infections. Immunol Allergy Clin North Am 37: 785–815, 2017. doi: 10.1016/j.iac.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peshkova IO, Fatkhullina AR, Mikulski Z, Ley K, Koltsova EK. IL-27R signaling controls myeloid cells accumulation and antigen-presentation in atherosclerosis. Sci Rep 7: 2255, 2017. doi: 10.1038/s41598-017-01828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pignolo RJ, Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res 26: 1685–1693, 2011. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- 138.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol 275: L1040–L1050, 1998. doi: 10.1152/ajplung.1998.275.6.L1040. [DOI] [PubMed] [Google Scholar]
- 139.Pulko V, Davies JS, Martinez C, Lanteri MC, Busch MP, Diamond MS, Knox K, Bush EC, Sims PA, Sinari S, Billheimer D, Haddad EK, Murray KO, Wertheimer AM, Nikolich-Žugich J. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat Immunol 17: 966–975, 2016. doi: 10.1038/ni.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation 124: 1783–1791, 2011. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rajamannan NM, Moura L. The lipid hypothesis in calcific aortic valve disease: the role of the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 36: 774–776, 2016. doi: 10.1161/ATVBAHA.116.307435. [DOI] [PubMed] [Google Scholar]
- 142.Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdörfer L, Korn T, Weiss S, Förster R, Prinz I. Interleukin-23-dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol 68: 2476–2486, 2016. doi: 10.1002/art.39732. [DOI] [PubMed] [Google Scholar]
- 143.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med, 2018. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJ, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RP, Libby P, Glynn RJ; CANTOS Trial Group . Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131, 2017. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 145.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 39: 3499–3507, 2018. doi: 10.1093/eurheartj/ehy310. [DOI] [PubMed] [Google Scholar]
- 146.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R; SEAS Investigators . Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 359: 1343–1356, 2008. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 147.Sage AP, Murphy D, Maffia P, Masters LM, Sabir SR, Baker LL, Cambrook H, Finigan AJ, Ait-Oufella H, Grassia G, Harrison JE, Ludewig B, Reith W, Hansson GK, Reizis B, Hugues S, Mallat Z. MHC Class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 130: 1363–1373, 2014. doi: 10.1161/CIRCULATIONAHA.114.011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saleh MA, Norlander AE, Madhur MS. Inhibition of Iinterleukin 17-A but not Interleukin-17F signaling lowers blood pressure and reduces end-organ inflammation in angiotensin ii-induced hypertension. JACC Basic Transl Sci 1: 606–616, 2016. doi: 10.1016/j.jacbts.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sanmarco LM, Eberhardt N, Ponce NE, Cano RC, Bonacci G, Aoki MP. New insights into the immunobiology of mononuclear phagocytic cells and their relevance to the pathogenesis of cardiovascular diseases. Front Immunol 8: 1921, 2018. doi: 10.3389/fimmu.2017.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181, 2010. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 151.Sawada H, Naito Y, Hirotani S, Akahori H, Iwasaku T, Okuhara Y, Miki K, Eguchi A, Mitsuno M, Miyamoto Y, Ohyanagi M, Tsujino T, Masuyama T. Expression of interleukin-33 and ST2 in nonrheumatic aortic valve stenosis. Int J Cardiol 168: 529–531, 2013. doi: 10.1016/j.ijcard.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 152.Schlotter F, Halu A, Goto S, Blaser MC, Body SC, Lee LH, Higashi H, DeLaughter DM, Hutcheson JD, Vyas P, Pham T, Rogers MA, Sharma A, Seidman CE, Loscalzo J, Seidman JG, Aikawa M, Singh SA, Aikawa E. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation 138: 377–393, 2018. doi: 10.1161/CIRCULATIONAHA.117.032291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schuster N, Krieglstein K. Mechanisms of TGF-β-mediated apoptosis. Cell Tissue Res 307: 1–14, 2002. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- 154.Schwartzenberg S, Meledin V, Zilberman L, Goland S, George J, Shimoni S. Low circulating monocyte count is associated with severe aortic valve stenosis. Isr Med Assoc J 15: 500–504, 2013. [PubMed] [Google Scholar]
- 155.Shimoni S, Bar I, Meledin V, Gandelman G, George J. Circulating regulatory T cells in patients with aortic valve stenosis: association with disease progression and aortic valve intervention. Int J Cardiol 218: 181–187, 2016. doi: 10.1016/j.ijcard.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 156.Shuvy M, Ben Ya’acov A, Zolotarov L, Lotan C, Ilan Y. Beta glycosphingolipids suppress rank expression and inhibit natural killer T cell and CD8+ accumulation in alleviating aortic valve calcification. Int J Immunopathol Pharmacol 22: 911–918, 2009. doi: 10.1177/039463200902200406. [DOI] [PubMed] [Google Scholar]
- 157.Skowasch D, Schrempf S, Wernert N, Steinmetz M, Jabs A, Tuleta I, Welsch U, Preusse CJ, Likungu JA, Welz A, Lüderitz B, Bauriedel G. Cells of primarily extra-valvular origin in degenerative aortic valves and bioprostheses. Eur Heart J 26: 2576–2580, 2005. doi: 10.1093/eurheartj/ehi458. [DOI] [PubMed] [Google Scholar]
- 158.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121: 1746–1755, 2010. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Somers P, Knaapen M, Kockx M, van Cauwelaert P, Bortier H, Mistiaen W. Histological evaluation of autophagic cell death in calcified aortic valve stenosis. J Heart Valve Dis 15: 43–47, 2006. [PubMed] [Google Scholar]
- 160.Spyridopoulos I, Martin-Ruiz C, Hilkens C, Yadegarfar ME, Isaacs J, Jagger C, Kirkwood T, von Zglinicki T. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell 15: 389–392, 2016. doi: 10.1111/acel.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis 43: 1143–1151, 2006. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 162.Šteiner I, Krbal L, Rozkoš T, Harrer J, Laco J. Calcific aortic valve stenosis: Immunohistochemical analysis of inflammatory infiltrate. Pathol Res Pract 208: 231–234, 2012. doi: 10.1016/j.prp.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 163.Šteiner I, Stejskal V, Žáček P. Mast cells in calcific aortic stenosis. Pathol Res Pract 214: 163–168, 2018. doi: 10.1016/j.prp.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 164.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 29: 630–634, 1997. doi: 10.1016/S0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 165.Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 18: 733–744, 2018. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 166.Szeto K, Pastuszko P, del Álamo JC, Lasheras J, Nigam V. Bicuspid aortic valves experience increased strain as compared to tricuspid aortic valves. World J Pediatr Congenit Heart Surg 4: 362–366, 2013. doi: 10.1177/2150135113501901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Takahashi K, Satoh M, Takahashi Y, Osaki T, Nasu T, Tamada M, Okabayashi H, Nakamura M, Morino Y. Dysregulation of ossification-related miRNAs in circulating osteogenic progenitor cells obtained from patients with aortic stenosis. Clin Sci (Lond) 130: 1115–1124, 2016. doi: 10.1042/CS20160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Taleb S, Tedgui A. IL-17 in atherosclerosis: the good and the bad. Cardiovasc Res 114: 7–9, 2018. doi: 10.1093/cvr/cvx225. [DOI] [PubMed] [Google Scholar]
- 169.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol 188: 4876–4884, 2012. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Toli K, Paraskevas KI, Poulakou MV, Agrogiannis G, Kavantzas N, Xanthopoulos V, Iliopoulos DG, Mantas I, Papachristodoulou A, Patsouris E, Mikhailidis DP, Perrea DN. Association between plasma levels and immunolocalization of cytokines in heart valve lesions: a possible target for treatment? Expert Opin Ther Targets 12: 1209–1215, 2008. doi: 10.1517/14728222.12.10.1209. [DOI] [PubMed] [Google Scholar]
- 171.Tsai CL, Chiu YM, Lee YJ, Hsieh CT, Shieh DC, Tsay GJ, Bau DT, Wu YY. Interleukin-32 plays an essential role in human calcified aortic valve cells. Eur Cytokine Netw 29: 36–47, 2018. [DOI] [PubMed] [Google Scholar]
- 172.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol 287: 134–145, 2005. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 173.Wald O, Weiss ID, Wald H, Shoham H, Bar-Shavit Y, Beider K, Galun E, Weiss L, Flaishon L, Shachar I, Nagler A, Lu B, Gerard C, Gao JL, Mishani E, Farber J, Peled A. IFN-gamma acts on T cells to induce NK cell mobilization and accumulation in target organs. J Immunol 176: 4716–4729, 2006. doi: 10.4049/jimmunol.176.8.4716. [DOI] [PubMed] [Google Scholar]
- 174.Walker JA, McKenzie ANJ. TH2 cell development and function. Nat Rev Immunol 18: 121–133, 2018. doi: 10.1038/nri.2017.118. [DOI] [PubMed] [Google Scholar]
- 175.Wang R, Chen W, Ma Z, Li L, Chen X. M1/M2 macrophages and associated mechanisms in congenital bicuspid aortic valve stenosis. Exp Ther Med 7: 935–940, 2014. doi: 10.3892/etm.2014.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wehner S, Buchholz BM, Schuchtrup S, Rocke A, Schaefer N, Lysson M, Hirner A, Kalff JC. Mechanical strain and TLR4 synergistically induce cell-specific inflammatory gene expression in intestinal smooth muscle cells and peritoneal macrophages. Am J Physiol Gastrointest Liver Physiol 299: G1187–G1197, 2010. doi: 10.1152/ajpgi.00452.2009. [DOI] [PubMed] [Google Scholar]
- 177.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation 114: 2065–2069, 2006. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 178.Winchester R, Wiesendanger M, O’Brien W, Zhang H-Z, Maurer MS, Gillam LD, Schwartz A, Marboe C, Stewart AS. Circulating activated and effector memory T cells are associated with calcification and clonal expansions in bicuspid and tricuspid valves of calcific aortic stenosis. J Immunol 187: 1006–1014, 2011. doi: 10.4049/jimmunol.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu B, Wang Y, Xiao F, Butcher JT, Yutzey KE, Zhou B. Developmental mechanisms of aortic valve malformation and disease. Annu Rev Physiol 79: 21–41, 2017. doi: 10.1146/annurev-physiol-022516-034001. [DOI] [PubMed] [Google Scholar]
- 180.Wu HD, Maurer MS, Friedman RA, Marboe CC, Ruiz-Vazquez EM, Ramakrishnan R, Schwartz A, Tilson MD, Stewart AS, Winchester R. The lymphocytic infiltration in calcific aortic stenosis predominantly consists of clonally expanded T cells. J Immunol 178: 5329–5339, 2007. doi: 10.4049/jimmunol.178.8.5329. [DOI] [PubMed] [Google Scholar]
- 181.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med 9: 4–14, 2009. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 182.Wu L, Van Kaer L. Natural killer T cells in health and disease. Front Biosci (Schol Ed) 3: 236–251, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wypasek E, Natorska J, Grudzień G, Filip G, Sadowski J, Undas A. Mast cells in human stenotic aortic valves are associated with the severity of stenosis. Inflammation 36: 449–456, 2013. doi: 10.1007/s10753-012-9565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wypasek E, Potaczek DP, Lamplmayr M, Sadowski J, Undas A. Interleukin-6 receptor Asp358Ala gene polymorphism is associated with plasma C-reactive protein levels and severity of aortic valve stenosis. Clin Chem Lab Med 52: 1049–1056, 2014. doi: 10.1515/cclm-2013-0606. [DOI] [PubMed] [Google Scholar]
- 185.Yang JH, Sakamoto H, Xu EC, Lee RT. Biomechanical regulation of human monocyte/macrophage molecular function. Am J Pathol 156: 1797–1804, 2000. doi: 10.1016/S0002-9440(10)65051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yap CH, Kim HS, Balachandran K, Weiler M, Haj-Ali R, Yoganathan AP. Dynamic deformation characteristics of porcine aortic valve leaflet under normal and hypertensive conditions. Am J Physiol Heart Circ Physiol 298: H395–H405, 2010. doi: 10.1152/ajpheart.00040.2009. [DOI] [PubMed] [Google Scholar]
- 187.Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, Furukawa K. Tumor necrosis factor-α accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther 337: 16–23, 2011. doi: 10.1124/jpet.110.177915. [DOI] [PubMed] [Google Scholar]
- 188.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol 168: 554–561, 2002. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 189.Zeng Q, Jin C, Ao L, Cleveland JC Jr, Song R, Xu D, Fullerton DA, Meng X. Cross-talk between the Toll-like receptor 4 and Notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. Circulation 126, Suppl 1: S222–S230, 2012. doi: 10.1161/CIRCULATIONAHA.111.083675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zeng Q, Song R, Fullerton DA, Ao L, Zhai Y, Li S, Ballak DB, Cleveland JC Jr, Reece TB, McKinsey TA, Xu D, Dinarello CA, Meng X. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc Natl Acad Sci USA 114: 1631–1636, 2017. doi: 10.1073/pnas.1619667114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhan Q, Song R, Li F, Ao L, Zeng Q, Xu D, Fullerton DA, Meng X. Double-stranded RNA upregulates the expression of inflammatory mediators in human aortic valve cells through the TLR3-TRIF-noncanonical NF-κB pathway. Am J Physiol Cell Physiol 312: C407–C417, 2017. doi: 10.1152/ajpcell.00230.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhan Q, Zeng Q, Song R, Zhai Y, Xu D, Fullerton DA, Dinarello CA, Meng X. IL-37 suppresses MyD88-mediated inflammatory responses in human aortic valve interstitial cells. Mol Med 23: 83–91, 2017. doi: 10.2119/molmed.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhou J, Zhu J, Jiang L, Zhang B, Zhu D, Wu Y. Interleukin 18 promotes myofibroblast activation of valvular interstitial cells. Int J Cardiol 221: 998–1003, 2016. doi: 10.1016/j.ijcard.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 194.Zizzo G, Cohen PL. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. J Immunol 190: 5237–5246, 2013. doi: 10.4049/jimmunol.1203017. [DOI] [PMC free article] [PubMed] [Google Scholar]