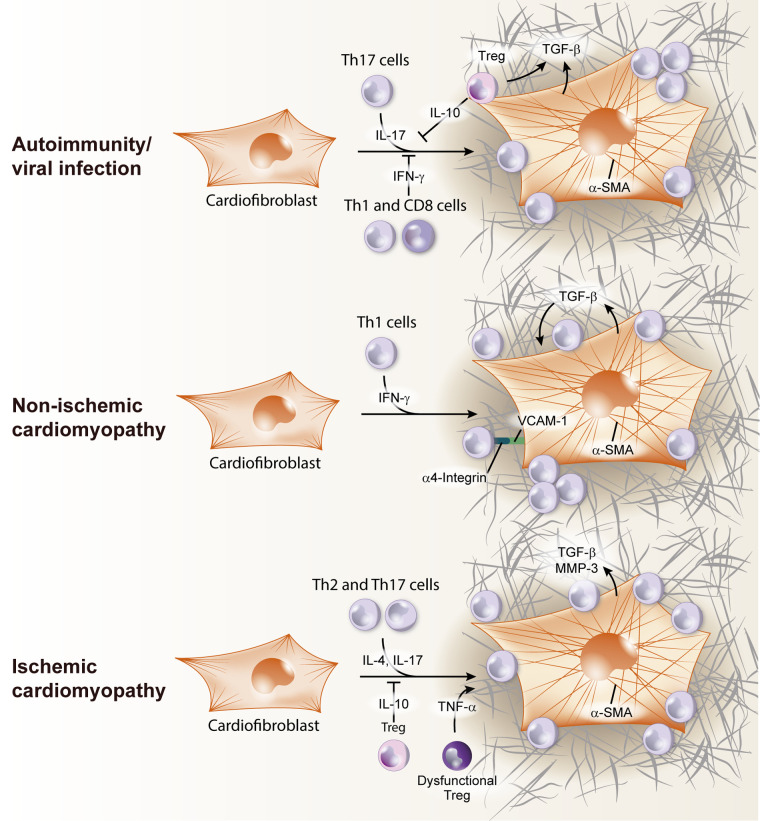
Fig. 3.
T-cell induction of cardiac fibroblast transition to myofibroblast in the heart. In viral infection/autoimmunity, Th17 cells produce IL-17, that lead to cardiac fibroblast activation and transforming growth factor-β (TGF-β) production. Treg can also release TGF-β and contribute to extracellular matrix remodeling, but T regulatory cells (Tregs) have a dual role, inhibiting Th17 response by IL-10 secretion. Cardiofibroblast activation can be inhibited by Th1/CD8 T-cell-produced IFN-γ. During nonischemic heart failure (HF), Th1 cells use α4-integrin to adhere to cardiac fibroblasts and contact is required for the transition to TGF-β producing myofibroblasts. In ischemic HF, Th2 and Th17 cells activate cardiac fibroblast by IL-17 and IL-4 release, stimulating TGF-β production and metalloproteinase 3 (MMP-3). This process was reported to be inhibited by IL-10 produced by Tregs. α-SMA, α-smooth muscle actin.