Figure 3.
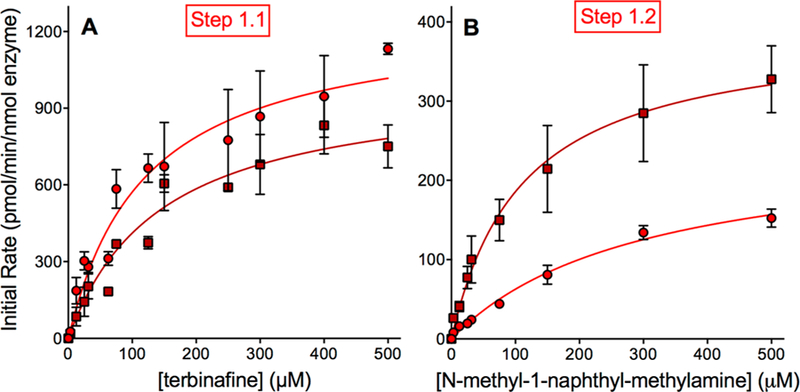
Steady-state kinetic profiles for terbinafine N-dealkylation in Pathway 1. CYP2C19 (○) and 3A4 (□) metabolism of terbinafine yielded kinetics for N-methyl-1-naphthyl methylamine (A) and TBF-A (not shown, see Results) in Pathway 1, as shown in Figure 1. The subsequent N-denaphthylation of N-methyl-1-naphthyl methylamine yielded kinetic profiles in (B). The sets of data were fit best to the Michaelis−Menten equation (p < 0.05), and the corresponding constants reported in Tables 1 and 2. A total of 12 experimental reactions were carried out for these studies. Error bars denote the standard deviations. Reaction conditions and data analyses were carried out as described in Experimental Procedures.
