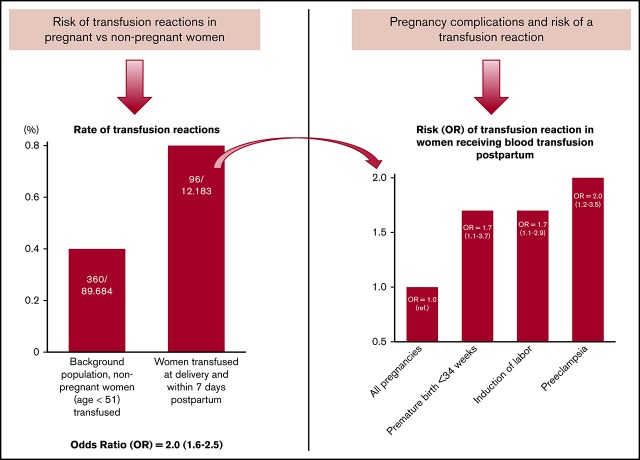
Key Points
Blood transfusions postpartum have an increased risk for transfusion reactions, especially in pregnancies complicated by preeclampsia.
Abstract
Postpartum hemorrhages with blood transfusions are increasing in many high-resource countries. Currently, up to 3% of all women receive blood transfusion postpartum. Most blood transfusions are safe and, in many cases, are lifesaving, but there are significant concerns about adverse reactions. Pregnancy is associated with higher levels of leukocyte antibodies and has a modulating effect on the immune system. Our objective was to investigate whether blood transfusions postpartum are accompanied by an increased risk for transfusion reactions (TRs) compared with transfusions given to nonpregnant women. We included all women who gave birth in Stockholm County, Sweden between 1990 and 2011. Data from the Swedish National Birth Registry were linked to the Stockholm Transfusion Database and included information on blood components administered and whether a TR occurred in women who received blood transfusions postpartum. Background controls were nonpregnant women who received blood transfusions during the study period. The study cohort consisted of 517 854 women. Of these, 12 183 (2.4%) received a blood transfusion. We identified 96 events involving a TR postpartum, giving a prevalence of 79 per 10 000 compared with 40 per 10 000 among nonpregnant women (odds ratio, 2.0; 95% confidence interval, 1.6-2.5). Preeclampsia was the single most important risk factor for TRs (odds ratio, 2.1; 95% confidence interval, 1.7-2.6). We conclude that special care should be taken when women with preeclampsia are considered for blood transfusion postpartum, because our findings indicate that pregnancy is associated with an increased risk for TRs.
Visual Abstract
Introduction
Postpartum hemorrhage is the most frequent cause of maternal mortality and morbidity worldwide.1 In high-resource countries, the number of women who require blood transfusions as a result of postpartum hemorrhage ranges from 2 to 30 per 1000 deliveries, and the figure continues to rise.2-5
Although the majority of blood transfusions are safe, adverse transfusion reactions (TRs) and transfusion-transmitted infections are a reality.6,7 TRs have different symptoms, depending on their pathophysiologies. The spectrum of symptoms ranges from mild, such as febrile or urticarial reactions, to severe; in some cases, reactions can be life-threatening. Deaths linked to TRs have been reported at a rate of 1 per every 100 000 transfused units.8
TRs can be dichotomized as immunological/nonimmunological or acute/delayed. They differ in frequency, definition, management, and risk profile.9,10 Knowledge of these adverse effects is essential before prescribing a blood transfusion and may, in some cases, result in opting for an alternative to blood transfusion. The most common TRs are the febrile nonhemolytic reaction and, since 2006, transfusion-related circulatory overload, with a registered frequency of 0.1 to 1.0 in 100 transfusions.10-13 The more serious reactions are rare and include anaphylactic TRs, septic TRs, acute hemolytic TRs, delayed hemolytic TRs, transfusion-related acute lung injury (TRALI), and transfusion-associated graft-versus-host disease.9,10,14,15
HLA and neutrophil antibodies are associated with TRs and most often cause mild nonhemolytic reactions with fever and chills; however, the development of TRALI has also been linked to leukocyte antibodies in the donor or the recipient.16,17 During pregnancy, the maternal immune system is altered with induced tolerance and immunization against fetal antigens. On the other hand, higher levels of HLA antibodies are observed in most pregnancies, especially in multiparous women.18 These circumstances may increase the risk of TRs during pregnancy.17,19 The prevalence of red cell, leukocyte, and platelet antibodies is known to increase in women with previous pregnancies17,20; however, the incidence of TRs in women receiving blood postpartum is not known.
The objective of our study was to assess the incidence and risk factors for postpartum TRs in women transfused with red blood cells (RBCs), plasma, or platelets postpartum.
Methods
In this retrospective population-based cohort study, we included all women who gave birth at >22 + 0 (22 weeks and 0 days) gestational weeks in Stockholm County between 1990 and 2011. By use of the mother’s personal identification number, data on pregnancies and deliveries from the National Medical Birth Registry were cross-linked to the Stockholm transfusion database (ProSang; Databyrån, Stockholm, Sweden). The National Medical Birth Registry, initiated in 1973, has been validated and is of high quality.21,22 All transfusions of blood components within Stockholm County have been registered in the Stockholm transfusion database since the beginning of 1980, with full coverage since 1985. According to the regulations of the National Board of Health, all transfusions must be reported. As a result, >99% of all transfused units are traceable to an individual through the system. The data include the personal identification numbers and blood group of donor and recipient, the unique serial number of the blood component, the time when the blood component was issued, whether the blood was transfused or returned, and whether a TR was reported. In Sweden, a National Hemovigilance Register began in 2005; however, in Stockholm County, TRs have been registered since 1980. Unexpected reactions that occurred in conjunction with a blood transfusion were reported directly by the clinicians involved, and the specific blood product was returned to the blood bank where the TR was entered into the transfusion database. The TRs were not registered by type, but they included acute febrile nonhemolytic mild allergic hypotensive acute hemolytic TRALI, septic reactions, posttransfusion purpura, anaphylactic reactions, and later-occurring transfusion-associated graft-versus-host disease. Transfusion-associated circulatory overload was included after 2006. Women with a TR postpartum were compared with the general pregnant background population and with nonpregnant women younger than 51 years of age in the Stockholm area who had received a transfusion during the period studied. The incidence of TRs may vary with age. Because most nonpregnant women who receive blood transfusions are older than the pregnant population, only women younger than 51 years of age were included in the background population.
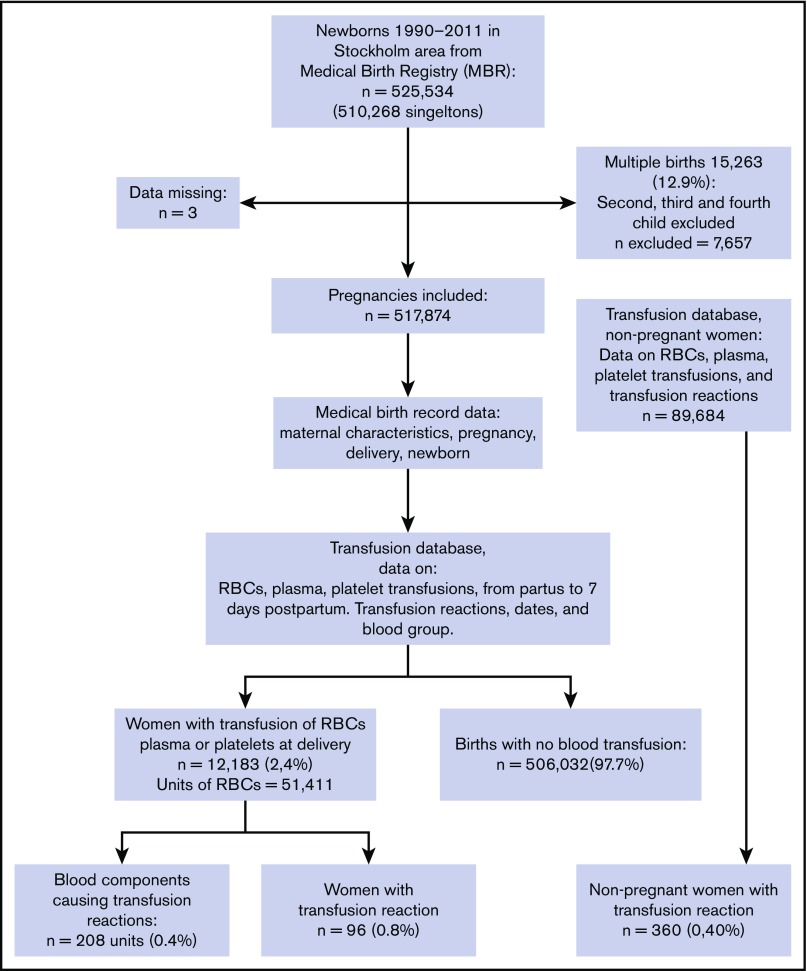
The final data were merged and anonymized. We identified women with transfusions of RBCs, plasma, or platelets and whether a TR was registered within 7 days of partus. The process of inclusion is illustrated in the flowchart in Figure 1.
Figure 1.
Flowchart for the study population.
The combined data set included information on age, ethnicity, body mass index (BMI), smoking, previous deliveries, prior cesarean sections, in vitro fertilization pregnancy, multiple pregnancies, date of delivery, diagnostic and procedural codes during pregnancy and delivery (using the International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision), mode of delivery, blood group, number of transfused units (of blood, plasma, or platelets), sex of the donor, and whether a TR occurred. Placenta previa and placenta accreta were combined into a single variable: placenta previa/accreta. We are presenting data for the women, not the newborn (ie, duplex pregnancies were treated as 1 case). Data on possible risk factors are presented separately for maternal and pregnancy variables (Table 2) and for transfusion-related variables (Table 3).
Table 2.
Maternal and pregnancy characteristics associated with a TR
| Risk factor | Total deliveries (n = 517 874) | Women transfused (n = 12 183) | TR cases (n = 96) | TR* crude OR | 95% CI | TR† adjusted‡ OR |
95% CI |
|---|---|---|---|---|---|---|---|
| Maternal characteristics | |||||||
| Age, y | |||||||
| ≤34 | 403 396 | 9 253 | 67 | 1.0 (ref) | |||
| 35-39 | 94 875 | 2 351 | 24 | 1.5 | 1.0-2.4 | 1.4 | 0.9-2.8 |
| ≥40 | 19 603 | 579 | 5 | 1.5 | 0.6-3.8 | 1.2 | 0.5-3.0 |
| Ethnicity | |||||||
| European | 433 170 | 9 637 | 72 | 1.0 (ref) | |||
| Non-European | 84 704 | 2 546 | 24 | 1.6 | 1.0-2.6 | 1.3 | 0.8-2.1 |
| Previous deliveries | |||||||
| 0 | 237 778 | 7 420 | 56 | 1.5 | 1.0-2.3 | 0.8 | 0.5-1.3 |
| 1 | 188 435 | 3 196 | 30 | 1.0 (ref) | ref | ||
| ≥2 | 91 661 | 1 567 | 10 | 0.7 | 0.3-1.4 | 0.6 | 0.3-1.2 |
| Previous CS | |||||||
| No | 474 886 | 10 735 | 84 | 1.0 (ref) | |||
| Yes | 42 988 | 1 448 | 12 | 1.6 | 0.9-2.9 | 1.1 | 0.5-2.1 |
| Pregnancy complications | |||||||
| Multiple pregnancy | |||||||
| No | 510 268 | 11 479 | 87 | ||||
| Yes | 7 606 | 704 | 9 | 6.9 | 3.5-13.8 | 1.4 | 0.7-2.8 |
| Placenta previa/accreta | |||||||
| No | 516 675 | 11 911 | 92 | 1.0 (ref) | |||
| Yes | 1 199 | 272 | 4 | 18.5 | 6.8-50.3 | 2.2 | 0.8-6.4 |
| Preeclampsia | |||||||
| No | 505 567 | 11 116 | 78 | 1.0 (ref) | |||
| Yes | 12 307 | 1 067 | 18 | 9.3 | 5.6-15.6 | 2.0 | 1.2-3.5 |
| Variables at delivery | |||||||
| Induction of labor | 53 936 | 2 284 | 29 | 3.7 | 2.4-5.8 | 1.7 | 1.1-2.9 |
| Mode of delivery | |||||||
| Spontaneous vaginal | 395 169 | 6 456 | 14 | 1.0 (ref) | |||
| Instrumental vaginal | 40 819 | 2 082 | 52 | 2.6 | 1.5-4.7 | 0.8 | 0.4-1.5 |
| CS | 81 886 | 3 645 | 30 | 2.9 | 1.8-4.7 | 0.8 | 0.5-1.3 |
| Placental disorders | |||||||
| Placental abruption | 1 328 | 229 | 4 | 17.0 | 6.2-46.2 | 2.6 | 0.9-7.3 |
| PPH and uterine atony | 12 080 | 1 730 | 14 | 8.4 | 4.7-14.8 | 1.1 | 0.6-1.9 |
| Newborn characteristics | |||||||
| Premature (≤34 + 0 gw) | 11 075 | 797 | 12 | 6.5 | 3.6-12.0 | 1.7 | 1.0-3.7 |
gw, gestational weeks; ref, reference.
Background = total deliveries.
Background = all women who received a blood transfusion postpartum (n = 12 183).
Adjusted for age, ethnicity, previous deliveries, previous CS, multiple pregnancy, placenta previa/accreta, preeclampsia, induction of labor, placental disorders, and premature birth ≤34 gw.
Table 3.
Transfusion characteristics among pregnant women with or without a TR
| Characteristics | No TR (n = 12 087) | TR (n = 96) | P |
|---|---|---|---|
| Transfusion entity and combinations | |||
| RBC units, median (IQR) | 2 (2-4) | 3 (2-5) | |
| Plasma units, median (IQR) | 2 (2-4) | 2 (2-5) | |
| 1 unit RBCs | 364 (3.0) | 21 (21.9) | <.001 |
| ≥10 units RBCs | 303 (2.5) | 6 (6.3) | .020 |
| RBCs only | 9 721 (80.4) | 56 (58.3) | <.001 |
| Plasma only | 278 (2.3) | 2 (2.1) | .888 |
| RBCs + plasma | 2 028 (16.8) | 37 (38.5) | <.001 |
| RBCs + plasma + platelets | 319 (2.6) | 13 (13.5) | <.001 |
| Transfusion characteristics | |||
| Blood group AB (mother) | 651 (5.4) | 7 (7.3) | .411 |
| Blood group A (mother) | 5 129 (42.4) | 37 (38.5) | .447 |
| Blood group B (mother) | 1 604 (13.3) | 13 (13.5) | .938 |
| Blood group O (mother) | 4 703 (38.9) | 39 (40.6) | .731 |
| Blood group RH+ (mother) | 10 395 (86.0) | 82 (85.4) | .869 |
| Donor characteristic | |||
| Female donor of RBCs, plasma* | 1 099 021 (47.1) | 106 (52.2) | .147 |
Unless otherwise noted, all data are n (%).
IQR, interquartile range.
Female donor of RBC and plasma units: comparing the background consisting of all nonpregnant women (2 331 584 units) with women with a TR postpartum (203 units).
Plasma components are not well standardized. In Sweden, fresh-frozen plasma or liquid plasma stored for up to 14 days was used during the study period. Cryoprecipitate was not used during the study period. Since 1999, universal leukocyte reduction has been used for all patients receiving blood components, with no special consideration regarding pregnancy. Beginning in 2006, only male donors were used in all plasma transfusions. Platelets were a pooled product from several donors or prepared from apheresis; preparation was the same in the pregnant and the nonpregnant populations.
The study was approved by the Regional Ethics Committee in Stockholm (2016/17-31/1).
Statistical analysis
All statistical calculations were performed using SPSS version 22 software (SPSS, Chicago, IL). Odds ratio (OR) was used as a measure of risk. Crude and adjusted ORs with 95% confidence intervals (CIs) were calculated using cross-tabulation and logistic regression analysis. TR was the dependent variable in the logistic regression analysis. Selected independent maternal variables with P < .1 were included in the multivariate analysis (age, ethnicity, prior deliveries, previous cesarean section, multiple pregnancy, preeclampsia, placenta previa/accreta, induction of labor, mode of delivery, placental disorders, and premature birth ≥ 34 + 0). Data on BMI were missing in almost one third of all pregnancies and were not used in the multivariate analysis.
Continuous variables were categorized where appropriate. The Student t test or the Mann-Whitney U test was used for normally and nonnormally distributed continuous data, and the χ2 test or Fisher’s exact test was used for categorical data, as appropriate. P < .05 was used to define statistical significance for all analyses.
Results
During the study period, 517 854 pregnancies (with 525 534 newborns) had been recorded and were included in the analysis (Figure 1). Three women whose medical birth registry data were missing were excluded. At the time of delivery, 510 268 women had singleton births and 7,606 (1.47%) woman had multiple births. Blood transfusion of ≥1 unit of RBCs from the time of delivery to 7 days postpartum occurred in 11 842 pregnancies (2.3%).
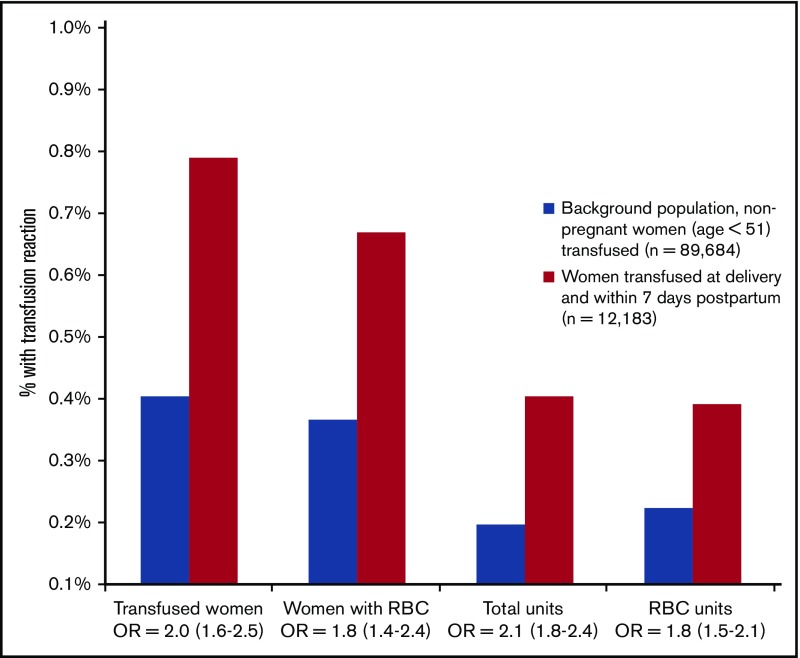
Among the 48 352 units of blood components transfused postpartum, 208 units (0.4%) were registered as having resulted in a TR. We found 96 women with a TR (79 per 10 000) among the 12 183 pregnant women receiving ≥1 transfused unit of RBCs, plasma, or platelets compared with 360 women with a TR (40 per 10 000) among the 89 684 nonpregnant women in the background population who had a blood transfusion (OR, 2.0; 95% CI, 1.6-2.5) (Figure 2). There was no difference in mean age between the pregnant population and the nonpregnant background population. The number of blood components was higher than the number of women registered with a TR because, in cases with simultaneous transfusion of RBCs, plasma, and platelets, it was impossible to identify which component caused the TR; hence, >1 component was registered.
Figure 2.
Frequency of reported TRs with corresponding OR (95% CI). Transfused women includes all women with RBC, plasma, or platelet transfusion. Total units is all units of RBCs, plasma, and platelets transfused.
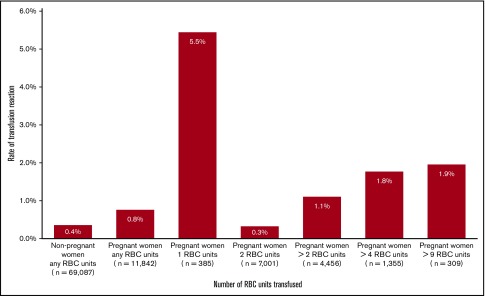
The unique event of a TR linked to an RBC unit was reported in 78 of the 11 842 (0.7%) pregnant women receiving an RBC transfusion compared with 252 of the 69 087 (0.4%) nonpregnant women who had an RBC transfusion during the same period. This indicates an 80% increased risk for a TR in women postpartum (OR, 1.8; 95% CI, 1.4-2.4) (Figure 2). The frequency of a TR was highest in women transfused with 1 unit of RBCs (5.5%; 21/385) and lowest in women transfused with 2 units of RBCs (0.3%; 23/7001). In women receiving massive transfusions (≥10 units of RBC within 24 hours), the TR rate was 1.9% (Figure 3).
Figure 3.
Rate (%) of TRs in relation to the number of transfused RBC units in pregnant and nonpregnant women.
Table 1 shows maternal and infant characteristics, including data on delivery, for the pregnant background population, women receiving ≥1 RBC transfusion, and women with a TR postpartum. Compared with all pregnancies involving a blood transfusion, pregnancies with a documented TR were more common in women with preeclampsia, induction of labor, placental complications, or a premature delivery at <34 weeks. The number of previous deliveries, age, or smoking did not increase the rate of a TR.
Table 1.
Maternal and newborn characteristics
| Characteristics | Background (n = 506 032) | ≥1 RBC (n = 11 842) | TR (n = 96) |
|---|---|---|---|
| Maternal characteristics | |||
| Age, y | |||
| <20 | 7 077 (1.4) | 171 (1.5) | 2 (2.1) |
| 20-34 | 387 323 (76.5) | 8 825 (74.5) | 65 (67.7) |
| 35-39 | 92 590 (18.3) | 2 285 (19.3) | 24 (25.0) |
| 40+ | 19 042 (3.8) | 561 (4.7) | 5 (5.2) |
| Mean (SD) | 31 (5.1) | 31 (5.3) | 32 (5.6) |
| Ethnicity (non-European) | 84 704 (16.7) | 2 468 (20.8) | 24 (25.0) |
| BMI, kg/m2 | |||
| <20 | 20 058 (5.9) | 499 (6.1) | 6 (9.4) |
| 20-30 | 301 834 (88.5) | 7 139 (87.5) | 52 (81.2) |
| 31-35 | 13 942 (4.1) | 364 (4.5) | 3 (4.7) |
| >35 | 5 283 (1.5) | 155 (1.9) | 3 (4.7) |
| Missing, n | 164 915 | 3 685 | 32 |
| Smoker | 50 041 (9.9) | 934 (7.9) | 9 (9.4) |
| Blood group O | 191 521 (37.8) | 4 618 (39.0) | 39 (40.6) |
| Previous deliveries | |||
| 0 | 230 570 (45.6) | 7 208 (60.9) | 56 (58.3) |
| 1 | 185 318 (36.6) | 3 117 (26.3) | 30 (31.3) |
| ≥2 | 90 144 (17.8) | 1 517 (12.8) | 10 (10.4) |
| Previous CS | 41 592 (8.2) | 1 396 (11.8) | 12 (12.5) |
| Diabetes mellitus | 1 894 (0.4) | 71 (0.6) | 1 (1.0) |
| Pregnancy and delivery related | |||
| Multiple pregnancy | 6 924 (1.4) | 682 (5.8) | 9 (9.4) |
| IVF | 8 708 (1.7) | 467 (3.9) | 4 (4.2) |
| Preeclampsia | 11 511 (2.3) | 796 (6.7) | 18 (18.8) |
| Labor induction | 51 701 (10.2) | 2 235 (18.9) | 29 (30.2) |
| Mode of delivery | |||
| Vaginal (spontaneous) | 388 950 (76.9) | 6 365 (53.7) | 52 (54.2) |
| Instrumental vaginal | 38 606 (7.6) | 2 075 (17.5) | 14 (14.6) |
| CS | 78 476 (15.5) | 3 402 (28.7) | 30 (31.3) |
| Placenta previa/accrete | 940 (0.2) | 259 (2.2) | 4 (4.2) |
| Placental abruption | 1 103 (0.2) | 225 (1.9) | 4 (4.2) |
| PPH/atonic | 10 354 (2.0) | 1 726 (14.6) | 14 (14.6) |
| Newborn characteristics | |||
| Premature (<34 + 0), wk | 10 387 (2.1) | 688 (5.8) | 12 (12.5) |
| Birth weight >4000 g | 90 697 (17.9) | 2 976 (25.1) | 21 (21.9) |
| Male sex | 259 958 (51.4) | 6 061 (51.2) | 51 (53.1) |
Unless otherwise noted, all data are n (%).
CS, cesarean section; IVF, in vitro fertilization; ; PPH, postpartum hemorrhage; SD, standard deviation.
In Table 2 we present crude and adjusted ORs with their 95% CIs for possible risk factors for a TR. In the multivariate regression analysis, only preeclampsia, induced labor, and premature birth at <34 weeks were significant factors (OR, 2.0; OR, 1.7; and OR, 1.7; respectively). There was no significant difference in risk regarding the proportion of female blood donors. The plasma or RBC donor was female in 52.2% of all cases among pregnant women and in 47.1% of all cases among women in the nonpregnant background population (Table 3). TRs were more common when a combination of blood components was administered (P < .001). There were no differences by blood group or frequency of erythrocyte antibodies in women with or without a TR. No deaths occurred among women with a TR.
Discussion
We found the incidence of TRs among women who received a postpartum transfusion of RBCs, plasma, or platelets to be 79 per 100 000 (0.8%), a doubled risk (OR, 2.0) compared with nonpregnant women (Figure 2). The postpartum risk of an adverse reaction after transfusions of RBCs only was increased by 80% (OR, 1.8); nevertheless, the overall risk remained low. Compared with the background pregnant population, women with preeclampsia (OR, 2.0), induced labor (OR, 1.7), or premature delivery (OR, 1.7) were at increased risk for a TR. The risk increased significantly when a combination of all 3 types of blood components was administered (Table 3). This might be explained, in part, by the higher risk of a TR from plasma and platelet units compared with RBC transfusions exclusively. On the other hand, there might also be an indication bias because sicker patients, with an increased risk for TRs, need broader transfusion support. The highest frequency of TRs (5.5%) was seen among women receiving only 1 unit of RBCs compared with women receiving massive blood transfusions, where TRs were registered at 2%. Massive blood transfusion postpartum is reported to occur in 2 to 9 per 10 000 deliveries (L.T., A.W., M.W., and P.G.L., manuscript submitted May 2019).3,23,24 The high prevalence of TRs in women receiving only a single unit of RBCs may have several explanations. The most probable reason is that further transfusions were avoided as soon as symptoms of an adverse TR appeared, as is recommended in Sweden.
We found no differences by donor sex or blood group. Our finding that female blood donors are not overrepresented among recipients who experience a TR is in contrast to other reports indicating an increased risk for TR in transfusions from female donors.25 Before the introduction of leukoreduced blood products and the use of predominantly male plasma, the risk of TRALI was reported to be sevenfold higher in plasma transfusions from female donors.25 Since 2008, plasma transfusions from female donors have not been used in Sweden, which might explain our findings. TRALI is very rare today.
TRs have been reported to occur in 0.15% to 3.7% of all blood transfusions, with the transfusion of platelets resulting in the highest prevalence.26,27 In a study from the United States, the overall rate of TRs was reported to be 0.27%, which is similar to our finding of 0.40% among nonpregnant women younger than 51 years of age.28 The wide range in prevalences could be due to differences in patient characteristics, reporting systems, and the criteria used to identify a TR, as well as variations in the methods used to prepare blood components.
We speculate that 1 possible explanation for the increased TRs seen in pregnancy is the higher levels of antibodies against HLA or human neutrophil antigen and other antigens expressed on leukocytes, platelets, and RBCs. Antibodies toward HLA are seen in 10% to 50% of all pregnant women and seem to increase with the number of pregnancies.17,29 However, we failed to note any increased risk in multipara, which contradicts the aforementioned data.
TRALI is now a rare clinical diagnosis; it is characterized by an increased rate of ventilation and acute hypoxemia, followed by a noncardiogenic pulmonary edema (with bilateral infiltrates on chest radiograph) during or within 6 hours of a blood transfusion.30 The mechanism of TRALI is not fully known, but the most accepted pathogenic theory is a 2-event model. In the first event, a clinical condition, such as critical illness, transplantation surgery, or infection, results in activation of the pulmonary endothelium and neutrophil sequestration, which enhances the sensitivity of a subsequent triggering signal. In the second event, the “primed” neutrophils are activated by a biological factor/signal in the blood product being transfused, resulting in damage to the pulmonary endothelium and leading to increased vascular permeability and pulmonary edema.31-33 Preeclampsia is related to endothelial dysfunction and could theoretically be an equivalent condition that activates the endothelium, as in the first event.34,35 When a later blood transfusion occurs, it might trigger a development similar to TRALI. Hypertensive disorders, such as preeclampsia, and increased levels of specific RBC antibodies during pregnancy have been associated with TRs.36,37 Our findings, indicating a twofold increased risk for TRs in women with preeclampsia, could be a result of endothelial damage. A study by Teofili et al reported a much higher increased risk for TRALI in women with a pregnancy-related hypertensive disorder (OR, 28), but they used a different study design specifically focusing on TRALI.36 The investigators retrospectively identified signs of possible TRALI among all women receiving postpartum blood transfusions; however, they only included women who had received ≥3 units of RBCs, which might explain their higher risk estimates.
Amniotic fluid embolism (AFE) is a severe pregnancy complication with a striking clinical resemblance to TRALI, usually presenting as an anaphylactoid reaction at delivery or shortly thereafter.38,39 Risk factors include abnormal placentation, induction of labor, and hypertensive pregnancy conditions (ie, the same as those for TRs).38,39 Thus, our findings are in agreement with the prior speculation that AFE might be caused by an incompatible feto-maternal blood transfusion triggering an acute hemolytic TR.40 Kobayashi suggested a broader definition of AFE as an anaphylactic/anaphylactoid syndrome of pregnancy.41
Strengths and weaknesses
A major strength of our study is its large size and the use of a single high-quality transfusion database rather than voluntary International Statistical Classification of Diseases coding in medical journals. In addition, we were able to compare rates of TRs between a large nonpregnant background population (0.4%) and a well-characterized pregnant population (0.8%) (P < .001), using the same reporting system and definition of TRs in a unified transfusion database. The nonpregnant background population was age matched to the pregnant population and consisted of all women younger than 51 years who had not received a blood transfusion between 1990 and 2011; however, our investigation does not have the advantages of a prospective study and is dependent on the thoroughness of the reporting clinicians. Another limitation is that we did not have access to the type of TR in each case, nor did we have data on transfusion history or ethical permission to enter each medical file. The registration of adverse events started in 1980, and it became mandatory to report severe cases after 2005. As a result, there might be some underreporting in the first half of the study period. In addition, there might be different reporting from pregnant women of which we have not been aware. However, our aim was to identify risk factors for TRs in women postpartum, comparing the incidence of TRs in a group of obstetric patients with all nonpregnant female transfusion recipients. A final weakness in evaluating TRs is the lack of internationally agreed-upon definitions; however, these limitations are likely to be shared by the TR cases and the background population in our study.
We found that TRs occurred twice as often among pregnant women as in the nonpregnant background population. Preeclampsia, induced labor, and preterm delivery were significant risk factors for TR, but we found no differences due to parity, donor gender, or blood group. Although most blood transfusions are safe and, in many cases, lifesaving, the risk of adverse TRs should be considered when deciding whether a blood transfusion is necessary.6,26,42 Our findings suggest heightened attention be paid when patients with preeclampsia are being evaluated for blood transfusions postpartum.
Acknowledgment
This work was supported in part by Södra Sjukvårdsregionen (grant REGSKANE-542301) (L.T.).
Authorship
Contribution: L.T. designed and performed the research, collected and analyzed data, performed statistical analysis, and wrote the manuscript; A.W. designed the research, collected and analyzed data, edited the manuscript, and approved the final submitted version of the manuscript; M.W. designed the research, analyzed data, edited the manuscript, and approved the final submitted version of the manuscript; and P.G.L. designed the research, collected and analyzed data, performed statistical analysis, edited the manuscript, and approved the final submitted version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lars Thurn, Department of Obstetrics and Gynecology, Skånes Universitetssjukvård, Region Skåne, SE-221 85 Lund, Sweden; e-mail: lars.thurn@ki.se.
References
- 1.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066-1074. [DOI] [PubMed] [Google Scholar]
- 2.Ekeroma AJ, Ansari A, Stirrat GM. Blood transfusion in obstetrics and gynaecology. Br J Obstet Gynaecol. 1997;104(3):278-284. [DOI] [PubMed] [Google Scholar]
- 3.Mhyre JM, Shilkrut A, Kuklina EV, et al. . Massive blood transfusion during hospitalization for delivery in New York State, 1998-2007. Obstet Gynecol. 2013;122(6):1288-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer MS, Berg C, Abenhaim H, et al. . Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449.e1-7. [DOI] [PubMed] [Google Scholar]
- 5.Balki M, Dhumne S, Kasodekar S, Carvalho JCA, Seaward G. Blood transfusion for primary postpartum hemorrhage: a tertiary care hospital review. J Obstet Gynaecol Can. 2008;30(11):1002-1007. [DOI] [PubMed] [Google Scholar]
- 6.Kuehnert MJ, Basavaraju SV, Moseley RR, et al. . Screening of blood donations for Zika virus infection - Puerto Rico, April 3-June 11, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(24):627-628. [DOI] [PubMed] [Google Scholar]
- 7.Zou S, Stramer SL, Dodd RY. Donor testing and risk: current prevalence, incidence, and residual risk of transfusion-transmissible agents in US allogeneic donations. Transfus Med Rev. 2012;26(2):119-128. [DOI] [PubMed] [Google Scholar]
- 8.Bolton-Maggs PHB. Serious hazards of transfusion - conference report: celebration of 20 years of UK haemovigilance. Transfus Med. 2017;27(6):393-400. [DOI] [PubMed] [Google Scholar]
- 9.Delaney M, Wendel S, Bercovitz RS, et al. ; Biomedical Excellence for Safer Transfusion (BEST) Collaborative . Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388(10061):2825-2836. [DOI] [PubMed] [Google Scholar]
- 10.Savage WJ. Transfusion reactions. Hematol Oncol Clin North Am. 2016;30(3):619-634. [DOI] [PubMed] [Google Scholar]
- 11.Sanders RP, Maddirala SD, Geiger TL, et al. . Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. Br J Haematol. 2005;130(5):781-787. [DOI] [PubMed] [Google Scholar]
- 12.Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44(1):16-24. [DOI] [PubMed] [Google Scholar]
- 13.Rogers MA, Rohde JM, Blumberg N. Haemovigilance of reactions associated with red blood cell transfusion: comparison across 17 countries. Vox Sang. 2016;110(3):266-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koussi A, Trachana M, Pliaki P, Gombakis N, Athanassiou-Metaxa M. Transfusion related acute lung injury syndrome (TRALI) in a patient with thalassaemia. Pediatr Blood Cancer. 2006;46(7):829-830, author reply 831. [DOI] [PubMed] [Google Scholar]
- 15.Pham HP, Shaz BH. Update on massive transfusion. Br J Anaesth. 2013;111(suppl 1):i71-i82. [DOI] [PubMed] [Google Scholar]
- 16.Curtis BR, McFarland JG. Mechanisms of transfusion-related acute lung injury (TRALI): anti-leukocyte antibodies. Crit Care Med. 2006;34(5 suppl):S118-S123. [DOI] [PubMed] [Google Scholar]
- 17.Triulzi DJ, Kleinman S, Kakaiya RM, et al. . The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49(9):1825-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerci Gurbuz B, Soyoz M, Ozkale Okyay D, Kilicaslan Ayna T, Pirim I. Comparison of anti-HLA antibody production according to gestational periods in pregnant women. Transplant Proc. 2017;49(3):464-466. [DOI] [PubMed] [Google Scholar]
- 19.Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178(6):3345-3351. [DOI] [PubMed] [Google Scholar]
- 20.De Clippel D, Baeten M, Torfs A, et al. . Screening for HLA antibodies in plateletpheresis donors with a history of transfusion or pregnancy. Transfusion. 2014;54(12):3036-3042. [DOI] [PubMed] [Google Scholar]
- 21.Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18(2):143-148. [DOI] [PubMed] [Google Scholar]
- 22.The National Board of Health and Welfare, Sweden. The Swedish Medical Birth Register—A Summary of Content and Quality. Stockholm, Sweden; 2003.
- 23.Ramler PI, van den Akker T, Henriquez DDCA, Zwart JJ, van Roosmalen J. Incidence, management and outcome of women requiring massive transfusion after childbirth in the Netherlands: secondary analysis of a nationwide cohort study between 2004 and 2006. BMC Pregnancy Childbirth. 2017;17(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green L, Knight M, Seeney FM, et al. . The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: a national cross-sectional study. BJOG. 2016;123(13):2164-2170. [DOI] [PubMed] [Google Scholar]
- 25.Chapman CE, Stainsby D, Jones H, et al. ; Serious Hazards of Transfusion Steering Group . Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. [DOI] [PubMed] [Google Scholar]
- 26.Hirayama F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. Br J Haematol. 2013;160(4):434-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolton-Maggs PH. Bullet points from SHOT: key messages and recommendations from the Annual SHOT Report 2013. Transfus Med. 2014;24(4):197-203. [DOI] [PubMed] [Google Scholar]
- 28.Sapiano MRP, Savinkina AA, Ellingson KD, et al. . Supplemental findings from the National Blood Collection and Utilization Surveys, 2013 and 2015. Transfusion. 2017;57(suppl 2):1599-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bux J, Jung KD, Mueller-Eckhardt G, Mueller-Eckhardt C. [Granulocyte-specific and HLA antibodies in pregnancy: incidence and clinical value]. Beitr Infusionsther. 1992;30:446-449. [PubMed] [Google Scholar]
- 30.Toy P, Popovsky MA, Abraham E, et al. ; National Heart, Lung and Blood Institute Working Group on TRALI . Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33(4):721-726. [DOI] [PubMed] [Google Scholar]
- 31.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34(5 suppl):S124-S131. [DOI] [PubMed] [Google Scholar]
- 32.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI). Br J Haematol. 2007;136(6):788-799. [DOI] [PubMed] [Google Scholar]
- 33.Fung YL, Silliman CC. The role of neutrophils in the pathogenesis of transfusion-related acute lung injury. Transfus Med Rev. 2009;23(4):266-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien M, Baczyk D, Kingdom JC. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, rather than extracellular vesicles. Sci Rep. 2017;7(1):5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin K, Audette MC, Parker JD, Kingdom JC. Mechanisms and clinical significance of endothelial dysfunction in high-risk pregnancies. Can J Cardiol. 2018;34(4):371-380. [DOI] [PubMed] [Google Scholar]
- 36.Teofili L, Bianchi M, Zanfini BA, et al. . Acute lung injury complicating blood transfusion in post-partum hemorrhage: incidence and risk factors. Mediterr J Hematol Infect Dis. 2014;6(1):e2014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchese M. Postpartum acute hemolytic transfusion reactions associated with anti-Lea in two pregnancies complicated by preeclampsia. Immunohematology. 2017;33(3):114-118. [PubMed] [Google Scholar]
- 38.Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, Knight M. Incidence, risk factors, management and outcomes of amniotic-fluid embolism: a population-based cohort and nested case-control study. BJOG. 2016;123(1):100-109. [DOI] [PubMed] [Google Scholar]
- 39.Knight M, Berg C, Brocklehurst P, et al. . Amniotic fluid embolism incidence, risk factors and outcomes: a review and recommendations. BMC Pregnancy Childbirth. 2012;12(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindqvist PG, Ajne G, Swahn ML, Westgren M. Can an incompatible foeto-maternal transfusion cause an acute maternal haemolytic transfusion reaction with fulminant disseminated intravascular coagulation? OA Case Reports. 2013;15(2):140. [Google Scholar]
- 41.Kobayashi H. Amniotic fluid embolism: anaphylactic reactions with idiosyncratic adverse response. Obstet Gynecol Surv. 2015;70(8):511-517. [DOI] [PubMed] [Google Scholar]
- 42.Rawn J. The silent risks of blood transfusion. Curr Opin Anaesthesiol. 2008;21(5):664-668. [DOI] [PubMed] [Google Scholar]