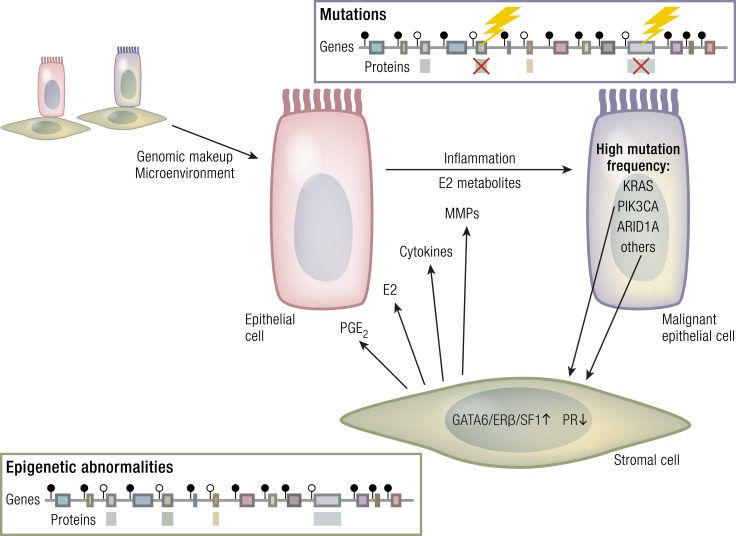
Figure 7.
Roles of endometriotic cell types with distinct abnormalities. Endometriotic stromal cells do not contain nonsynonymous base pair alterations (mutations) but display extensive epigenetic defects that regulate the expression or silencing of genes. Specific patterns of DNA methylation (●) or demethylation (○) give rise to the suppression or overproduction of specific proteins. In endometriotic stromal cells, GATA6, ERβ, and SF1 proteins are overproduced, whereas PR is suppressed, leading to progesterone resistance. These changes collectively cause the accumulation of inflammatory and tissue-remodeling substances, including PGE2, E2, cytokines, and matrix metalloproteinases (MMPs). In contrast, portions of the eutopic endometrial or endometriotic epithelial cells accumulate tumor driver mutations  that disrupt or change the function of critical proteins, including Kirsten rat sarcoma homolog (KRAS), phosphatidylinositol-3-kinase, catalytic α polypeptide (PIK3CA), AT-rich interactive domain-containing protein 1A (ARID1A), and numerous other oncogenes or tumor suppressors. Extraordinarily high concentrations of E2 and its metabolites in the ovary and stroma-derived inflammation may contribute to the accumulation of epithelial mutations. [Chronic inflammation is suspected to cause mutagenesis and carcinogenesis in other tissues such as Barrett’s esophagus and esophageal cancer (208).] The relative contributions of endometriotic stromal vs epithelial cells to the development of pelvic endometriosis are currently unknown. The effects of each cell type on the acquisition of genome-wide epigenetic defects or mutagenesis are also not known.
that disrupt or change the function of critical proteins, including Kirsten rat sarcoma homolog (KRAS), phosphatidylinositol-3-kinase, catalytic α polypeptide (PIK3CA), AT-rich interactive domain-containing protein 1A (ARID1A), and numerous other oncogenes or tumor suppressors. Extraordinarily high concentrations of E2 and its metabolites in the ovary and stroma-derived inflammation may contribute to the accumulation of epithelial mutations. [Chronic inflammation is suspected to cause mutagenesis and carcinogenesis in other tissues such as Barrett’s esophagus and esophageal cancer (208).] The relative contributions of endometriotic stromal vs epithelial cells to the development of pelvic endometriosis are currently unknown. The effects of each cell type on the acquisition of genome-wide epigenetic defects or mutagenesis are also not known.