Abstract
Background:
Using text messaging for vaccine safety monitoring, particularly for non-medically attended events, would be valuable for pandemic influenza and emergency vaccination program preparedness. We assessed the feasibility and acceptability of text messaging to evaluate fever and wheezing post-influenza vaccination in a prospective, observational, multi-site pediatric study.
Methods:
Children aged 2–11 years old, with an emphasis on children with asthma, were recruited during the 2014–2015 influenza season from three community-based clinics in New York City, and during the 2014–2015 and 2015–2016 seasons from a private practice in Fall River, Massachusetts. Parents of enrolled children receiving quadrivalent live attenuated (LAIV4) or inactivated influenza vaccine (IIV4) replied to text messages assessing respiratory symptoms (day 3 and 7, then weekly through day 42), and temperature on the night of vaccination and the next seven nights (day 0–7). Missing data were collected via diary (day 0–7 only) and phone. Phone confirmation was obtained for both presence and absence of respiratory symptoms. Reporting rates, fever (T ≥ 100.4 °F) frequency, proportion of wheezing and/or chest tightness reports captured via text message versus all sources (text, phone, diary, electronic health record) and parental satisfaction were assessed.
Results:
Across both seasons, 266 children were analyzed; 49.2% with asthma. Parental text message response rates were high (>70%) across sites. Overall, fever frequency was low (day 0–2: 4.1% [95% confidence interval (CI) 2.3–7.4%]; d3–7: 6.7% [95% CI 4.1–10.8%]). A third (39.2%) of parents reported a respiratory problem in their child, primarily cough. Most (88.2%) of the 52 wheezing and/or chest tightness reports were by text message. Most (88.1%) participants preferred text messaging over paper reporting.
Conclusions:
Text messaging can provide information about pediatric post-vaccination fever and wheezing and was viewed positively by parents. It could be a helpful tool for rapid vaccine safety monitoring during a pandemic or other emergency vaccination program.
Conclusions:
Trial registration: clinicaltrials.gov Identifier: .
Keywords: Influenza, Wheeze, Asthma, Vaccination, Influenza vaccination, Vaccine safety, Text message, SMS
1. Introduction
The Advisory Committee on Immunization Practices (ACIP) recommends influenza vaccination for all individuals ≥6 months-old [1]. Understanding influenza vaccine safety is important for annual vaccination programs and pandemic preparedness. Influenza vaccine is the only vaccine recommended annually, with formulations often changing yearly [2]. While seasonal vaccine safety patterns are usually similar, unexpected adverse events may arise. For example, the 2010–2011 trivalent inactivated influenza vaccine was associated with an increased febrile seizure risk in young children [3].
Although following a large number of vaccinated people prospectively for post-licensure vaccine-associated adverse events can be labor-intensive and expensive, we and others have successfully used text messaging- a scalable, low-cost method- for this purpose [4–8]. Currently, 95% of American adults have a cell phone [9]. Cell phone use is thought to be higher in harder-to-reach low-income populations [10] that may not be included in vaccine safety monitoring programs relying on information from managed care organizations [11]. Text messaging is particularly useful for monitoring of non-medically attended events, such as fever, since it allows patients or caregivers to report symptoms directly. This may be important in an influenza pandemic when information may need to be gathered rapidly and simultaneously from large numbers of vaccinated people nationally, but visits to medical facilities may be limited. Some important issues remain in the use of text messaging for monitoring vaccine-associated adverse events. First, adverse events may occur several weeks post-vaccination, but previous pediatric studies have focused primarily on the more immediate 7–10 days post-vaccination. Second, unlike in Australia [12], text messaging has not been used to date in the United States to assess vaccine safety outcomes at multiple sites, but multi-site vaccine safety monitoring would have value for pandemic and emergency vaccination program preparedness. Third, although wheezing is not considered a safety concern after IIV, accuracy of text messaging to capture respiratory events may be pertinent for monitoring safety of live attenuated influenza vaccination (LAIV) [13]. During our study period, ACIP recommended LAIV preferentially for healthy 2- through 8-year olds for the 2014–15 season; for 2015–16 season there was no preferential recommendation, but LAIV was an acceptable option for use in healthy children [14,15].
This study’s primary goal was to assess feasibility and acceptability of text messaging to assess wheezing prospectively from day 0 (vaccination day) through day 42 post-vaccination in children aged 2–11 years-old receiving quadrivalent live attenuated (LAIV4) or inactivated (IIV4) influenza vaccine. We hypothesized that (1) at least 80% of wheezing symptoms reported via text message would be verified by phone interview, in those able to be reached, and medical record review for those with a visit; (2) response rates to text message queries regarding wheezing symptoms would be higher on days 3 and 7 post-vaccination than on days 14–42; and (3) at least 80% of parents would have a high level of satisfaction with using text messaging to report wheezing occurring post-vaccination. A secondary objective was to assess feasibility of monitoring post-vaccination wheezing and fever using text messaging at multiple sites. We hypothesized that text message response rates would not differ by more than 10 percentage points between sites.
2. Materials and methods
This prospective observational study was conducted during the 2014–2015 (Year 1) and 2015–2016 (Year 2) influenza seasons, in collaboration with the Centers for Disease Control and Prevention (CDC). During the 2014–2015 season, children were recruited from three community-based clinics affiliated with New York-Presbyterian Hospital/Columbia University Medical Center (CUMC) in New York City. These sites serve a primarily Latino, publicly-insured population and share an electronic health record (EHR) system with the hospital. During the 2014–2015 and 2015–2016 seasons, children were recruited from a private general pediatric practice in Fall River, Massachusetts affiliated with Boston Medical Center (BMC) that has its own EHR system. Each child also had a parent or guardian participate in the study. All text messages for both sites were sent from a centralized program to the parent (or guardian) participating in the study. Vaccination decisions were made by the patients’ health care provider and caregivers, and all influenza vaccines administered were the quadrivalent forms. The influenza vaccine strain composition differed in the two seasons for both an A and B strain. CUMC and BMC’s Institutional Review Boards (IRB) approved the study; CDC relied on the CUMC approval.
Children were eligible for enrollment if they (1) were 2–11 years-old, (2) were receiving their first or second influenza dose of that season, (3) their parent had a cell phone with text messaging capabilities, and (4) their parent spoke English or Spanish at CUMC sites or English at the BMC site. Exclusion criteria included (1) any chronic medical condition considered a contraindication or precaution for LAIV (with the exception of asthma/wheezing history) [14], (2) current/recent (<2 weeks) asthma exacerbation, (3) oral or other systemic steroid use in the preceding 2 weeks, (4) temperature (T) ≥100.4 °F at vaccination, (5) antipyretic administration within 6-hours pre-vaccination or stated intent to use prophylactic antipyretics, (6) parental inability to read or send text messages, and (7) sibling or child already enrolled in either season. Receipt of other vaccines was not an exclusion criterion.
2.1. Study procedures
Parents provided consent and completed an intake form, including demographic information and parent-reported child history of asthma/reactive airway disease (RAD)/recurrent wheezing. Study staff reviewed text message procedures. Parents received and were trained with a temporal artery thermometer [16], and were given a paper diary in a pre-addressed/pre-stamped envelope to return after the first 7-day observation period.
Parents were asked to take their child’s temperature each evening from the day of vaccination (day 0) through the next 7 days, or at any time during those days if the child felt febrile. Parents were sent an interactive text message (in English or Spanish, based on participant language choice) nightly, asking the highest temperature, time taken, name and time of any antipyretics given, and care sought. Respiratory questions were sent on days 3, 7, 14, 21, 28, 35 and 42 (none, wheeze, cough, and/or chest tightness). 1f respiratory symptoms were reported, additional information was prompted including medications and care visits. Unanswered text messages were re-sent 20 minutes later to prompt a response.
The centralized text messaging program had built-in messages sent back for an unexpected reply (e.g. T < 95 °F or >106 °F degrees) with instructions to correct the error. Study staff reviewed messages daily initiating contact with non-responders to collect missing data, and with responders reporting respiratory symptoms to confirm them. Parents that reported either no respiratory symptoms on d3–d21 texts or no symptoms some days and failed to report other days, were called soon after d21 to confirm the child had no symptoms. A telephone exit survey was administered to all participants after the last text message was sent on d42 to confirm absence or presence of respiratory symptoms occurring on d28–d42 and assess parental satisfaction.
Vaccinations given at enrollment and during d1–d42 post-vaccination, as well as all healthcare visits (ambulatory care, emergency department and hospital) between d0–d42 post-vaccination, were abstracted from the EHR. Chart documentation of history of asthma/RAD/recurrent wheezing, as well as associated medications, were abstracted. Abstracted data were used by two separate investigators per site to adjudicate whether the child had chart documentation of asthma exacerbation and/or asthma medication prescription documented within 12 months before enrollment (i.e., recent history), or asthma/RAD/ recurrent wheezing diagnosis not meeting criteria as above (i.e., remote history), or no history of asthma exacerbation or medication prescription. Adjudication discrepancies were reviewed and a final decision made by the entire research team.
2.2. Data analysis
Response rates to d3 and d7 text messages were compared to response rates sent weekly from days 14–42 using a paired t-test. A scale from 0 to 1 was calculated in which 0 was responding no days, 1 was defined as responding all days, and numbers between 0 and 1 represented the ratio of number of days responded per number of days messages were sent. This was then multiplied by 100 to result in a percent.
We described fever reported via text (any: T ≥ 100.4 °F; moderate to severe: T ≥ 102.2 °F) for d0–2 and d3–7 separately. These analyses included participants who reported on all days of the reporting period (d0–2, d3–7 separately) as well any child with fever even if the parent did not report all days in period. The d0–2 window was selected based on the increased fever risk previously observed on day 2 post-LAIV vaccination [17], and the known day 0–1 fever risk window after IIV [18].
To assess accuracy, we noted how many reports of wheezing and/or chest tightness were reported by any source (text, phone, diary [diary available for days 3 and 7 only], or EHR). We calculated the proportion of episodes captured via text message. For those who texted and we were able to reach by phone, we categorized each report via text message as concordant or discordant (texted respiratory symptom confirmed or not by phone). Results were tabulated separately for cough. We also describe the proportion of those with wheezing and/or chest tightness overall, by vaccine type and by history of asthma/RAD/recurrent wheezing.
A convenience sample size for this feasibility study was set to be at least 150 children and their caregivers in year 1 and 100 in year 2. Although asthma/RAD/recurrent wheezing was not an eligibility criterion, the goal was to enroll approximately 70% of children with this history in order to increase the chances for respiratory events.
3. Results
Two-hundred and sixty-eight children and their parent/guardian were enrolled: 168 in 2014–2015 and 100 in 2015–2016. Two participants were excluded from analysis (Fig. 1). Most children (72.9%) received IIV4 (Table 1). Demographic characteristics differed by recruitment site (Supplemental Table 1). The proportion of children with parent-reported history of asthma/RAD/recurrent wheezing was 32.5% in 2014–2015 (enrollment began in December) and 69.0% in 2015–2016 (enrollment began in October).
Fig. 1. Study enrollment flow.
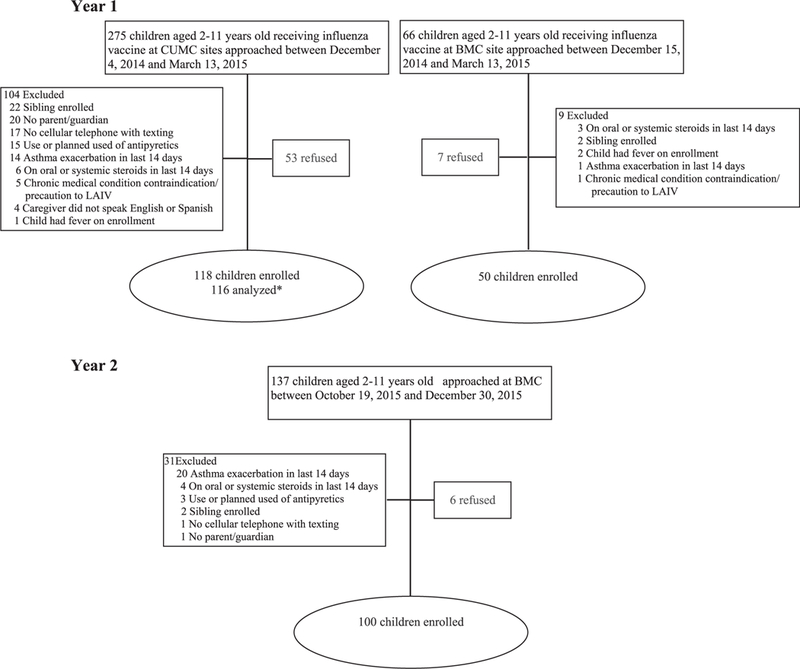
*Two participants removed by investigators after enrollment
• One child traveled outside U.S. on day 3 and parent did not have text messaging service out of the country
• One parent reported on sibling of enrolled child. The sibling was too young to be eligible for the study.
LATV4: Quadrivalent Live Attenuated Influenza Vaccine, IIV4: Quadrivalent Inactivated Influenza Vaccine
CUMC: Columbia University Medical Center; BMC: Boston Medical Center
Table 1.
Characteristics of study population.
| Year 1 | Year 2 | Study total | |
|---|---|---|---|
| (n = 166) | (n = 100) | (n = 266) | |
| Sex | |||
| Male | 95 (57.2%) | 55 (55.0%) | 150 (56.4%) |
| Female | 71 (42.8%) | 45 (45.0%) | 116(43.6%) |
| Age | |||
| 2 to <5 years | 75 (45.2%) | 28 (28.0%) | 103 (38.7%) |
| 5 to <8 years | 47 (28.3%) | 25 (25.0%) | 72 (27.1%) |
| 8 to <12 years | 44 (26.5%) | 47 (47.0%) | 91 (34.2%) |
| Latino | 101 (60.8%) | 14 (14.0%) | 115 (43.2%) |
| Race | |||
| Black/African American | 27 (16.3%) | 6 (6.0%) | 33 (12.4%) |
| White | 70 (42.2%) | 84 (84.0%) | 154 (57.9%) |
| Asian | 1 (0.6%) | 1 (1.0%) | 2 (0.8%) |
| Native American | 4 (2.4%) | 1 (1.0%) | 5 (1.9%) |
| Multiracial | 40 (24.1%) | 7 (7.0%) | 47 (17.7%) |
| Other | 24 (14.5%) | 1 (1.0%) | 25 (9.4%) |
| Asthma history overalla | 62 (37.3%) | 69 (69.0%) | 131 (49.2%) |
| History ≤12 months | 48 (28.9%) | 64 (64.0%) | 112 (42.1%) |
| History >12 months | 14 (8.4%) | 5 (5.0%) | 19 (7.1%) |
| Language caregiver most comfortable | |||
| English | 108 (65.1%) | 100 (100%) | 208 (78.2%) |
| Spanish | 58 (34.9%) | 0 (0%) | 58 (21.8%) |
| Insurance | |||
| Commercial | 24 (14.5%) | 39 (39.0%) | 63 (23.7%) |
| Public | 141 (84.9%) | 60 (60.0%) | 201 (75.6%) |
| Uninsured | 1 (0.6%) | 1 (1.0%) | 2 (0.8%) |
| Unlimited text message plan | 153 (93.9%) | 98 (98.0%) | 251 (95.4%) |
| Receive text messages daily | 158 (95.8%) | 100 (100%) | 258 (97.4%) |
| Vaccine type | |||
| LAIV4 | 54 (32.5%) | 18 (18.0%) | 72 (27.1%) |
| IIV4 | 112 (67.5%) | 82 (82.0%) | 194 (72.9%) |
| LAIV4 | |||
| LAIV4 alone | 41 (75.9%) | 12 (66.7%) | 53 (73.6%) |
| LAIV4 with other vaccines | 13 (24.1%) | 6 (33.3%) | 19 (26.4%) |
| IIV4 | |||
| IIV4 alone | 87 (77.7%) | 62 (75.6%) | 149 (76.8%) |
| IIV4 with other vaccines | 25 (22.3%) | 20 (24.4%) | 45 (23.2%) |
Year 1 2014–2015 (two sites), Year 2 2015–2016 (one site).
LAIV4: Quadrivalent Live Attenuated Influenza Vaccine, IIV4: Quadrivalent Inactivated Influenza Vaccine.
Asthma history <12 months includes documentation of asthma exacerbation and/or asthma medication prescription documented within 12 months before enrollment (i.e., recent history). Asthma history >12 months indicates has an asthma/RAD/ recurrent wheezing diagnosis but does not meet criteria as above (i.e., remote history).
Chart adjudication identified 131 (49.2%) children with a history of asthma/RAD/recurrent wheezing. Nearly all children with an asthma history received IIV4. Most (105/112; 93.8%) children with a chart-adjudicated history of asthma/RAD/recurrent wheezing in the last 12 months also had parent-reported asthma medication use in the last 12 months. Most (105/111; 94.6%) children with parent-reported asthma medication use in the last 12 months also had a recent chart history, and the remaining had a remote chart history.
Three (1.1%) participants requested to stop messages and two (0.8%) lost text message or phone service during the 42 days observation period. Half (53.0%) returned the diary in Year 1 and 78.0% in Year 2. Individual text message response rates were above 70% on all days across all sites (Fig. 2) though rates varied by day. Mean response rate for those who had text messages delivered was 88% ± 25% overall and was similar between the study period d3–7 versus d14–42 (90% ± 25% vs. 88% ± 27%; p = .26). Mean response rate was significantly higher at the BMC-affiliated site (95% ± 15% vs. 79% ± 32%; p <.001). Due to demographic differences by sites, results were stratified. When stratified by site, there were no differences in response rates by age, gender, preferred language, education level, text message plan or utilization of the respondent, or age, sex, race, ethnicity, insurance status of the child. In addition, across both sites combined, all demographic sub-groups had response rates of ≥80% with the exception of those who did not have unlimited texting (76% ± 36%) or did not text daily at baseline (74% ± 31%).
Fig.2. Response rates to text messages day 0 (day of vaccination) to day 42.
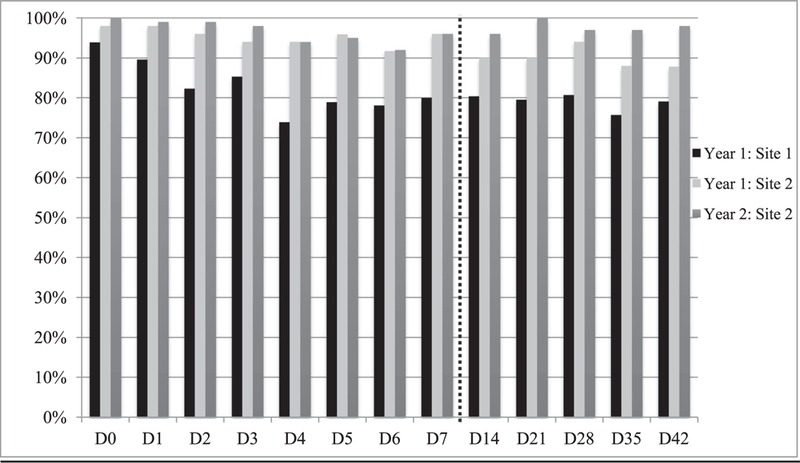
Site 1: Columbia University Medical Center
Site 2: Falls River (Boston Medical Center)
3.1. Fever and respiratory symptoms
The majority of fever and respiratory symptom data were collected via text message at both sites (Fig. 3). Fever frequency was low and similar between IIV4 and LAIV4 groups. Day 0–2 fever for both years combined was 4.1% (95% CI 2.3–7.4%) (LAIV4 2.9% [95% CI 0.8–10.1%]; IIV4 4.6% [95% CI 2.3–8.8%]). Fever frequency from d3–7 was also low: 6.7% (95% CI 4.1–10.8%) (LAIV4 3.6% [95% CI 1.0–12.1%]; IIV4 7.7% [95% CI 4.6–12.8%]). There was no statistically significant difference in day 0–2 fever among those with (1.7%) and without other vaccines (4.9%) administered at enrollment (p = .27), but there was a statistically significant difference for day 3–7 fever (16.7% with vs. 3.5% without other vaccines, p = .001). Of the 9 children with d3–7 fever who also received another vaccine, one received LAIV4 with other vaccines (Measles/Mumps/Rubella (MMR)-Varicella, Diphtheria/Tetanus/acellular Pertussis (DTaP)-inactivated polio vaccine (IPV), Hepatitis B, Hepatitis A) and eight received IIV4 with other vaccines ([MMR-Varicella (n = 2); DTaP-IPV (n = 2); Varicella, DTaP-IPV (n = 1); Hepatitis A (n = 1); Tdap, MenACWY-CRM (n = 1) and Hepatitis A, Haemophilus influenzae type b (n = 1)].
Fig. 3. Primary data source for (A) temperature data and (B) respiratory symptom data in study year 1 (Y1) and year 2 (Y2).
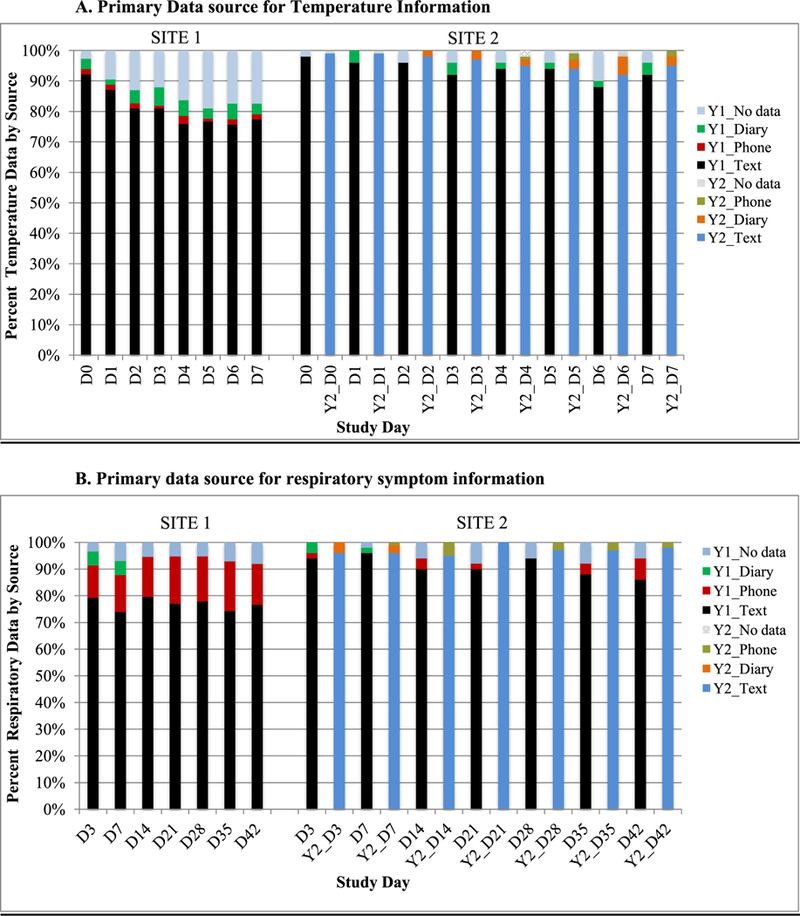
Primary data source categorized as text if had text information available, diary if no text information was available, phone if no text or diary information was available. Diary was available only for days 3 and 7.
Site 1 : Columbia University Medical Center, Site 2: Falls River (Boston Medical Center)
Overall, 39.2% of parents (37.2% at CUMC and 40.7% at BMC) who responded to at least one respiratory text message reported a problem (primarily cough). A total of 52 episodes of wheezing and/or chest tightness were reported during the study period; 27 episodes in Year 1 and 25 in Year 2. The highest frequencies of episodes were on day 7 (3.5%) and day 14 (3.9%).
Most episodes were reported via text message (88.5% overall, 89.5% CUMC and 87.9% BMC). Of the 35 episodes reported via text message for which we were able to reach the parent for confirmation, all but one were confirmed (97.1%).
Overall, 13.7% of children (95% CI 10.1–18.4%) had at least one episode of wheezing and/or chest tightness. Proportions were higher for IIV4 but not significantly (LAIV4 7.1% [95% CI 3.1–15.7%]; IIV4 16.1% [95% CI 11.6–22.0%](p = .061). A higher proportion of children with a recent history of asthma/wheezing/RAD on chart adjudication had wheezing and/or chest tightness [24.3% (95% CI 17.3–33.0%)] versus those who had a remote history [5.3% (95% CI 0.9–24.6%)]. Proportions were similar by vaccine type in those with a recent history (LAIV4 25.0% [95% CI 4.6–69.9%]; IIV4 24.3% [95% CI 17.2–33.2%]). Only 4 of these children received LAIV4. There was no difference in proportion of children with wheezing/chest tightness among those who did (9.4%) or did not (15.2%) have other vaccines administered at enrollment (p = .24).
There were 103 episodes of cough reported in Year 1 and 83 in Year 2 by text message, phone and/or diary. Most (86.6%) were reported via text message. Of the 141 episodes reported via text message for whom we were able to reach the parent for confirmation, all were confirmed.
Few episodes of wheeze and/or chest tightness resulted in medical visits (3 of 27, 11.1% in Year 1; 6 of 25, 24.0% in Year 2). Similarly, few episodes of cough alone resulted in medical visits (8 of 86, 9.3%) in Year 1; 7 of 71, 9.9% in Year 2). In Year 1 and 2 combined, 56 participants had at least one medical visit for any reason in the 42 days post-vaccination (13 emergency department, 45 primary care, 7 specialty care, 1 elective hospitalization).
3.2. Parental survey
Most parents completed the exit survey (83.6% CUMC, 94.7% BMC). Nearly all (99.6%) surveyed were satisfied taking part in the study (76.9% very satisfied, 22.7% satisfied) and would be very likely to recommend it to other families (87.4%). Most (96.6%) would be willing to take part in a future study using text messaging to follow their child’s health (79.8% very willing, 16.8% somewhat willing). Most (88.1%) preferred text messaging over paper reporting. Nearly 1 in 5 (19.2%) parents reported that taking part in the study positively affected their views on vaccine safety, and only 0.8% reported a potentially negative effect.
4. Discussion
We demonstrated in this novel study that text messaging is a reliable method to identify post-vaccination respiratory symptoms both in the more immediate seven days post-vaccination as well as through 42 days post-vaccination with most episodes- both medically attended and non-medically attended- being captured by text message. It also showed acceptability with high response rates and parental satisfaction. Finally, it was a successful demonstration of a central text messaging program with inclusion of a geographically distant health facility with no previous experience with text message monitoring. Response rates were high across sub-populations, which may be important for planning future pandemic or emergency vaccination safety programs.
Wheezing is considered to be an important outcome of interest in vaccine safety assessments [19], and there was a specific concern about wheezing after LAIV [17,20,21]. As noted above, LAIV was in use during the seasons our study was conducted 2014–15 and 2015–16). Subsequently, the ACIP recommended that LAIV should not be used in the U.S. during 2016–17 and 2017–18 influenza seasons, due to concerns about its effectiveness [1,22]. However, LAIV continues to be recommended and used in some other countries such as Canada and United Kingdom [23,24]. We saw no difference in wheezing/chest tightness by vaccine type in children with a recent asthma/RAD/recurrent wheeze history, but very few of those children received LAIV since there was a precaution against the use of LAIV in children with asthma or recurrent wheezing. Other studies have shown that IIV does not significantly increase asthma exacerbations immediately after vaccination in children over three years of age [25,26], and our proportion of wheezing/chest tightness episodes were similar to published data [25]. In our study, the majority of parent-reported wheezing and/or chest tightness episodes did not result in medically-attended visits, highlighting the importance of methods to collect data directly from participants as a complement to EHR monitoring. While these events may be considered as medically less serious since they do not require medical care, they may affect parental perceptions of vaccine safety [27–31]. Being able to assess a fuller complement of both medically-attended and non-medically-attended adverse events would be advantageous when evaluating a new vaccine.
While there could have been a potential concern that having parents directly report vaccine safety data may make them feel less safe about a vaccine, we found the opposite was true. This is similar to our findings in pregnant women for whom taking part in text message monitoring led to a perceived increase in positive views about vaccine safety [32]. Two potential reasons for this positive effect are that, first, parents are more aware that vaccines are carefully monitored [33,34], and, second, that as parents pay more attention in the post-vaccination period they realize their child did not experience any health issues.
A number of lessons were also learned about using text messaging for vaccine adverse event monitoring. First, while response rates drop off when texting daily for a number of days in a row, as seen in our previous studies [4,5], sending messages weekly for up to 6 weeks appears to be well-tolerated with sustained response rates. Second, it is helpful to build into the text messaging error messages that ask for clarification to cut down on erroneous responses, as well as to have unanswered text messages re-sent after 20 minutes to help prompt a response. For example, in response to the first message of the temperature cascade sent on day 0, an automated message was triggered 6.4% of the time to clarify a response; 88.2% resulted in correction of the initial response. In addition, a repeat message was triggered 27.1% of the time due to lack of response; 91.7% responded with data. Finally, specific to screening for asthma, we found that parent report of asthma medication use within the last 12 months was a fairly accurate indicator (>90%) of a chart-adjudicated recent asthma/RAD/recurrent wheeze history, which may be helpful for future studies.
This observational study had a number of limitations. While there was potential for underreporting, most episodes of wheezing and/or chest tightness were reported via text message. We could not reach all parents by phone to confirm symptoms in year one, and respiratory symptoms were confirmed by phone but not by medical exam. However, if a system such as this were used in a pandemic most episodes would also not be verified by a provider examination. There is previous evidence that caregiver report of lower respiratory symptoms in young children is sensitive, particularly when reporting wheeze [35]. Of note, if text messaging were to be used in a large scale post-licensure study, a text message report could be used to trigger a study visit, if desired. An additional limitation is that limiting the denominator by data reporting levels may have increased fever and wheezing rates, but overall rates were low. Finally, while the study being conducted at two geographically distant and demographically distinct sites is a strength, it may not be generalizable to other locations.
5. Conclusion
This study demonstrates the feasibility and acceptability of using text messaging to assess pediatric respiratory symptoms after influenza vaccination through 6 weeks post-vaccination. The results also illustrate the capacity to monitor post-vaccination fever and wheezing across more than one site, which could be helpful during an influenza pandemic or other emergency vaccination program.
Supplementary Material
Acknowledgements
We would like to thank Theresa Harrington MD MPH TM and Oidda Museru RN, MSN, MPH from the Centers for Disease Control and Prevention (CDC) for their technical contributions to the study, New York-Presbyterian Hospital for its support of the EzVac Immunization Information System, and the New York-Presbyterian Hospital Ambulatory Care Network and its staff and patients. We would like to thank Luis Alba, Zuleika Parra Valencia, Ormarys Castellanos, and Ameriangel Roman from Columbia University and Mary Banks from Boston Medical Center for their help in this study.
M.S. Stockwell is a co-investigator but receives no financial support for an unrelated, investigator-initiated grant from the Pfizer Medical Education Group. The other authors have no conflicts of interests or financial disclosures.
Source of funding
This study was supported through the Clinical Immunization Safety Assessment (CISA) Project Contract No. 200-2012-53665-0004 from the Centers for Disease for Control and Prevention (CDC).
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2017.10.073.
Disclaimer
The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices - United States, 2017–18 influenza season. MMWR Recomm Rep 2017;66(2):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gerdil C The annual production cycle for influenza vaccine. Vaccine 2003;21(16):1776–9. [DOI] [PubMed] [Google Scholar]
- [3].Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010–2011. Vaccine 2012;30(11):2024–31. [DOI] [PubMed] [Google Scholar]
- [4].Stockwell MS, Broder K, LaRussa P, et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr 2014;168(3):211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stockwell MS, Broder KR, Lewis P, et al. Assessing fever frequency after pediatric live attenuated versus inactivated influenza vaccination. J Pediatric Infect Dis Soc. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pillsbury A, Cashman P, Leeb A, et al. Real-time safety surveillance of seasonal influenza vaccines in children, Australia, 2015. Euro Surveill 2015;20(43). [DOI] [PubMed] [Google Scholar]
- [7].Westphal DW, Williams SA, Leeb A, Effler PV. Continuous active surveillance of adverse events following immunisation using SMS technology. Vaccine 2016;34(29):3350–5. [DOI] [PubMed] [Google Scholar]
- [8].U.S. Department of Health and Human Services. Using health text messages to improve consumer health knowledge, behaviors, and outcomes: an environmental scan. Rockville (MD): U.S. Department of Health and Human Services; 2014. [Google Scholar]
- [9].Mobile Fact Sheet, Pew Research Center; 2017. Available at <http://www.pewinternet.org/fact-sheet/mobile/> [accessed on April 14, 2017].
- [10].Blumberg SJ, Luke JV. Reevaluating the need for concern regarding noncoverage bias in landline surveys. Am J Public Health 2009;99(10):1806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moro PL, Li R, Haber P, Weintraub E, Cano M. Surveillance systems and methods for monitoring the post-marketing safety of influenza vaccines at the Centers for Disease Control and Prevention. Expert Opin Drug Saf 2016;15(9):1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].SmartVax. Available at <http://www.smartvax.com.au> [accessed on September 27, 2017].
- [13].Centers for Disease Control and Prevention. Institute of Medicine Reports on Vaccine Safety. Available at <http://www.cdc.gov/vaccinesafety/research/iomreports/index.html> [accessed on April 14, 2017].
- [14].Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) - United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014;63(32):691–7. [PMC free article] [PubMed] [Google Scholar]
- [15].Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 influenza season. MMWR Morb Mortal Wkly Rep 2015;64(30):818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Greenes DS, Fleisher GR. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr Adolesc Med 2001;155(3):376–81. [DOI] [PubMed] [Google Scholar]
- [17].Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007;356(7):685–96. [DOI] [PubMed] [Google Scholar]
- [18].Rowhani-Rahbar A, Klein NP, Dekker CL, et al. Biologically plausible and evidence-based risk intervals in immunization safety research. Vaccine 2012;31(1):271–7. [DOI] [PubMed] [Google Scholar]
- [19].Marangu D, Kovacs S, Walson J, et al. Wheeze as an adverse event in pediatric vaccine and drug randomized controlled trials: a systematic review. Vaccine 2015;33(41):5333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ambrose CS, Dubovsky F, Yi T, Belshe RB, Ashkenazi S. The safety and efficacy of live attenuated influenza vaccine in young children with asthma or prior wheezing. Eur J Clin Microbiol Infect Dis 2012;31(10):2549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tennis P, Toback SL, Andrews EB, McQuay LJ, Ambrose CS. A US postmarketing evaluation of the frequency and safety of live attenuated influenza vaccine use in nonrecommended children younger than 5years: 2009–2010 season. Vaccine 2012;30(42):6099–102. [DOI] [PubMed] [Google Scholar]
- [22].Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65(5):1–54. [DOI] [PubMed] [Google Scholar]
- [23].Flu Plan Winter; 2017. Available at <https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/600532/annual_flu_plan_2017to2018.pdf> [accessed on October 11. 2017].
- [24].Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2017–2018. Available at <https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2017-2018.html> [accessed on October 11, 2017].
- [25].Cates CJ, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev 2013(2):CD000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Quach C Vaccinating high-risk children with the intranasal live-attenuated influenza vaccine: the Quebec experience. Paediatr Respir Rev 2014;15(4):340–7. [DOI] [PubMed] [Google Scholar]
- [27].Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics 2010;125(4):654–9. [DOI] [PubMed] [Google Scholar]
- [28].Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015;33(3):459–64. [DOI] [PubMed] [Google Scholar]
- [29].Grant VJ, Le Saux N, Plint AC, et al. Factors influencing childhood influenza immunization. CMAJ 2003;168(1):39–41. [PMC free article] [PubMed] [Google Scholar]
- [30].Healy CM, Montesinos DP, Middleman AB. Parent and provider perspectives on immunization: are providers overestimating parental concerns? Vaccine 2014;32(5):579–84. [DOI] [PubMed] [Google Scholar]
- [31].Stockwell MS, Irigoyen M, Andres Martinez R, Findley SE. Failure to return: parental, practice, and social factors affecting missed immunization visits for urban children. Clin Pediatr (Phila) 2014;53(5):420–7. [DOI] [PubMed] [Google Scholar]
- [32].Stockwell MS, Cano M, Jakob K, et al. Feasibility of text message influenza vaccine safety monitoring during pregnancy. Am J Prev Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Byington CL. Vaccines: can transparency increase confidence and reduce hesitancy? Pediatrics 2014;134(2):377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dube E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother 2013;9(8):1763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Samet JM, Cushing AH, Lambert WE, et al. Comparability of parent reports of respiratory illnesses with clinical diagnoses in infants. Am Rev Respir Dis 1993;148(2):441–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


