Figure 1.
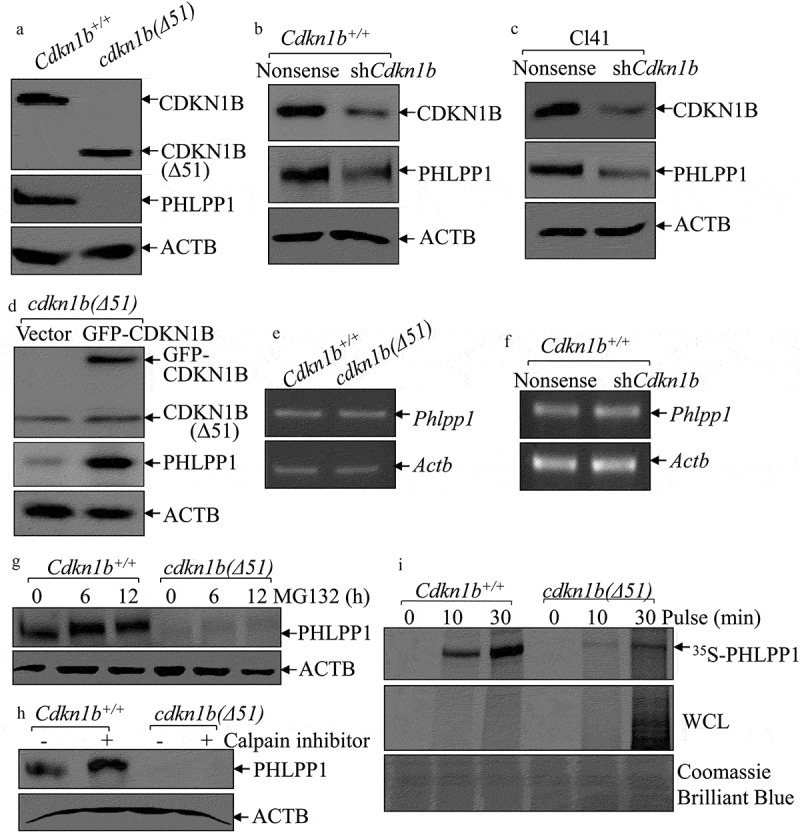
CDKN1B promoted PHLPP1 expression at protein translational level. (a–d) Western blots (WB) were used to determine the protein levels of PHLPP1 in Cdkn1b+/+ vs. cdkn1b(Δ51) cells (a), Cdkn1b+/+(Nonsense) vs. Cdkn1b+/+(shCdkn1b) (b), Cl41(Nonsense) vs. Cl41(shCdkn1b) (c), and cdkn1b(Δ51)(Vector) vs. cdkn1b−/-(Δ51)(GFP-CDKN1B) (d). ACTB was used as a protein loading control. (e,f) RT-PCR was applied to compare the Phlpp1 mRNA levels in Cdkn1b+/+ vs. cdkn1b(Δ51) (e) and Cdkn1b+/+(Nonsense) vs. Cdkn1b+/+(shCdkn1b) (f). Actb was used as an internal loading control. (g) Cdkn1b+/+ and cdkn1b(Δ51) cells were treated with MG132 for the indicated times. The cell extracts were then subjected to western blots to determine PHLPP1 protein accumulation. ACTB was used as a protein loading control. (h) Cdkn1b+/+ and cdkn1b(Δ51) cells were treated with Calpain inhibitor for 24 h. The cell extracts were then subjected to western blot analyses of the new PHLPP1 protein accumulation. ACTB was used as a protein loading control. (i) Newly synthesized PHLPP1 protein in Cdkn1b+/+ and cdkn1b(Δ51) cells was monitored by 35S-labeled methionine/cysteine pulse assay, as described in the section of ‘Materials and Methods’. WCL, whole cell lysate. Coomassie Brilliant Blue staining was used as a protein loading control.
