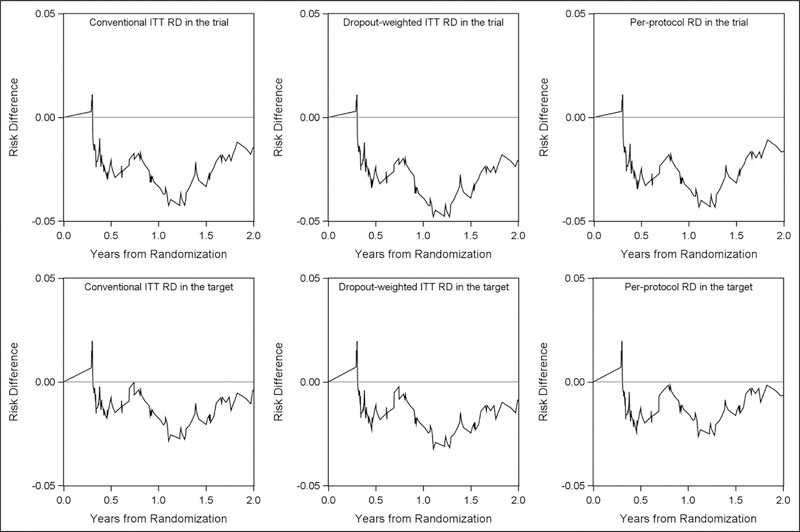
Figure 3.
Risk difference comparing four-drug arm to three-drug arm over 2 years (naïve ITT risks, dropout-weighted ITT risks, per-protocol risks that accounted for dropout and protocol deviations in the A5095 trial and in the target population). ITT: intention-to-treat; RD: risk difference; target: target population.
Upper panels represent risk differences over 2 years in the A5095 trial with the left panel depicting the conventional ITT risk difference under the independent censoring assumption, middle panel depicting the ITT risk difference that accounted for dropout, and the right panel depicting per-protocol risk difference that accounted for dropout and protocol deviation. Lower panels represent risk differences over 2 years in the target US recently HIV-diagnosed population with the left panel depicting the conventional ITT risk difference that only accounted for sampling bias, middle panel depicting the ITT risk difference that accounted for dropout and sampling bias, and the right panel depicting per-protocol risk difference that accounted for dropout, protocol deviation, and sampling bias.