Figure 4.
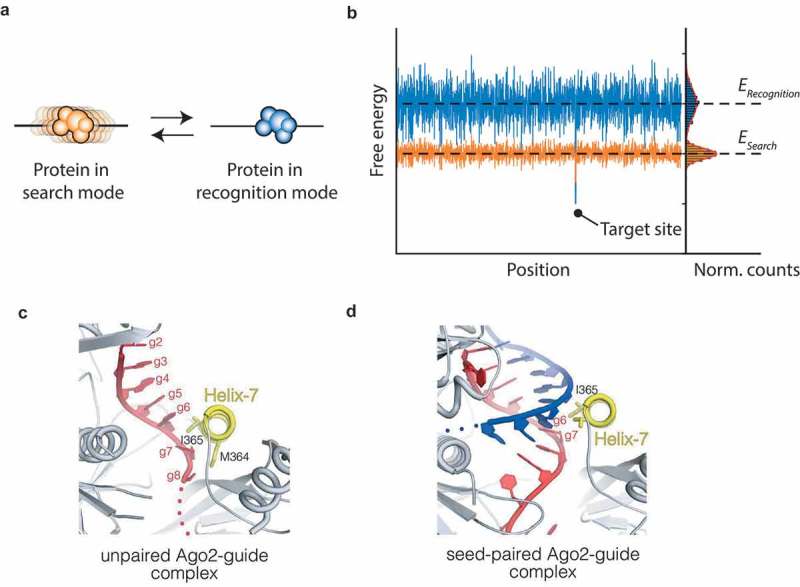
Argonaute undergoes a conformational change, as required by the speed-stability paradox.
(a) The speed stability paradox posited by Slutsky et al. In the search mode (orange), the protein is able to diffuse laterally without encountering significant energy barriers. Once it encounters a potential target site (indicated by the deeper energy level in the binding energy landscape right), it may switch to a recognition mode (blue). In this mode, the specificity of the protein is increased and the protein will not diffuse. (b) The energy landscape as encountered by a protein in the search mode (orange) and a protein in the recognition mode (blue). In the search mode, the landscape that the protein encounters is shallow and the variance in energy levels is small. The deeper energy levels in the recognition mode prevent the protein from diffusing laterally. At a potential target site, it’s more likely for the protein to switch from a search mode to a recognition mode, since the energy level of the former is higher than the latter. (c) Close-up view of the seed region shows the pre-formed helix of nt 2–6. Helix-7 disrupts base stacking by intercalating itself between g6 and g7 [66]. (PDB ID: 4OLA) (d) Close-up view of the seed region in the event of fully base-paired seed. Helix-7 undergoes a conformational change here, docking into the minor groove of the seed-paired complex [66]. (PDB ID: 4W5O). Permission has been obtained for the above figures. Copyright 2017 John Wiley and Sons.
